Overvåkingsprogrammet for sykdommene bonamiose og marteiliose i flatøsters og blåskjell utføres av Havforskningsinstituttet på oppdrag fra Mattilsynet. Det ble i 2021 hentet skjell fra ett dyrkingsanlegg for østers og en vill, kommersielt utnyttet østersbestand som også brukes som kilde til østers i et restaureringsprosjekt i Nederland, og ett blåskjellanlegg. Prøvene ble samlet inn i de periodene prevalensen av parasittene Bonamia spp. og Marteilia spp. er vist å være høyest i smittede bestander. Det er ikke observert unormal dødelighet verken vår eller høst. Bonamia ostreae / B. exitiosa ble ikke påvist. Parasitten Marteilia sp. ble for første gang påvist i ville blåskjell, Mytilus edulis, på Bømlo i 2016, på Tysnes i 2019 og i Sunnfjord i 2020. I 2021 ble Marteilia sp. påvist i enda en poll på Bømlo og i Solund. Funnene vil bli videre beskrevet i et pågående forskningsprosjekt. Alle funn av Marteilia i blåskjell i Norge er så langt Marteilia pararefringens, som antas å være spesifikk for blåskjell. Resultatene fra overvåkingen vil bli fulgt opp med en utvidet prøvetaking av ville blåskjellbestander i 2022 – 2023 i regi av forskningsprosjektene. Det foreslås en revisjon av overvåkingsprogrammet, søknad om etablering av fristatus for Bonamia spp i hele landet og Marteilia refringens i norsk flatøsters.
The surveillance and control programme for bonamiosis and marteiliosis in European flat oysters, Ostrea edulis, and blue mussels, Mytilus sp. in Norway in 2021
Report series:
Rapport fra havforskningen 2022-7
ISSN: 1893-4536
Published: 15.03.2022
Updated: 27.04.2022
Project No.: 14538
On request by: Mattilsynet
Reference: Odd Arne Brimsøe
Research group(s):
Smittespredning og sykdom
Program:
Miljøeffekter av akvakultur
Approved by:
Research Director(s):
Geir Lasse Taranger
Program leader(s):
Terje Svåsand
Norsk sammendrag
Summary
The surveillance programme is carried out by the Institute of Marine Research according to a contract with the Norwegian Food Safety Authority. In 2021, samples were collected from one oyster farm, one wild, commercially exploited oyster population which is also used as a source of oysters for a restoration project off the coast of The Netherlands, and one mussel farm. Samples were collected in April/May and in October, to be able to detect Bonamia sp. and Marteilia sp. during the periods when the potential prevalence is at the highest. No abnormal mortalities were observed during the surveillance. Bonamia ostreae / B. exitiosa were not detected. The parasite Marteilia sp. was detected for the first time in mussels, (Mytilus edulis) collected at Bømlo, western Norway in 2016, Tysnes in 2019, and Sunnfjord in 2020. In 2021 Marteilia sp. was found in another site at Bømlo and in Solund. The new detections will be further described in an on-going research project. All isolates found so far are M. pararefringens, which is regarded specific for mussels. The work will be continued in 2022, linked to research on the distribution of M. pararefringens in wild mussels. We propose a revision of the surveillance programme, application for disease free status for Bonamia spp. and Marteilia refringens in Norwegian flat oysters.
1 - Introduction
The surveillance programme for bonamiosis and marteiliosis in European flat oysters, Ostrea edulis , and blue mussels, Mytilus sp.
The surveillance programme for bonamiosis and marteiliosis in European flat oysters, Ostrea edulis , and blue mussels, Mytilus sp. is carried out by the Institute of Marine Research according to a contract with the Norwegian Food Safety Authority. The overall aim is to gain knowledge on the health situation of farmed Norwegian oysters and mussels, with an emphasis on notifiable diseases. There is a connection between farmed and wild populations, due to the geographically widespread and extensive nature of the bivalve industry. Some oyster farmers collect wild seed or half-grown oysters for on-growth in farms. The work therefore requires a tight link between the surveillance programme and the on-going research projects, sampling in commercially exploited wild populations as well as the building of a time-series of data on wild stocks. After the detection of Marteilia sp. in 2016, we increased the effort to study this case, including distribution and parasite life cycle. This report gives a brief overview of the present situation, results from 2021 and the prospects for the work in 2022.
Norwegian flat oyster, Ostrea edulis , are used in re-stocking projects
here is a growing interest in the re-establishment of wild oyster beds in Northern Europe, and initiatives united in the NORA project ( https://noraeurope.eu/ ) has developed a protocol on how to select, treat and seed oysters on some of the historical oyster beds. The Institute of Marine Research (IMR) is collaborating with this project, with contributions to their biosecurity manual (https://nativeoysternetwork.org/wp-content/uploads/sites/27/2020/11/ZSL00161%20Biosecurity%20Handbook%20ONLINE.pdf) and linking the selection of potential oyster source population to the on-growing health monitoring and research. The restoration of wild oyster beds is dependent on the availability of flat oysters free from Bonamia spp. and Marteilia refringens ( Sas et al . 2020 ) . Both the oyster farming industry and re-stocking projects therefore focus on where to find naïve flat oyster populations that are free from Bonamia spp, as well as other pathogens that may affect the populations. In the present situation – and after the re-occurrence of Bonamia ostreae in Limfjorden, Denmark, in 2014 – safe sources of oysters can probably only be found in Sweden and Norway. Norwegian populations of European flat oysters have been monitored since 1989 and are considered free from notifiable diseases ( Mortensen et al . 2016; 2020 ). Oysters from Hafrsfjord in Rogaland are being used in re-stocking trials in the North Sea. Oysters from this area are included in the surveillance program, and the stocks in Hafrsfjord is monitored annually.
Marteilia pararefringens sp. nov. in blue mussels, Mytilus edulis in Norway
The blue mussel, Mytilus sp, populations are changing, and there are numerous reports on an un-explained “disappearance” or mortalities from many Nordic areas. It is not known to which extent diseases play a role in these changes. The wild mussel beds are not monitored on a regular basis, and there is a limited control of mussel farms, using traditional longline cultivation based on the collection of wild seed. Marteilia pararefringens has been found in blue mussels, Mytilus edulis, in four traditional heliothermic marine oyster lagoons, two wild populations and one mussel farm in western Norway (see Figure 1 and report from 2020 ( Mortensen & Skår 2021 )). The effect of M. pararefringens on the mussel populations is the subject of an on-going research project and will be reported and published as the project progresses.
2 - Material and methods
The surveillance and following work were performed according to EU regulations 2016/429, 2017/625 and 2020/689. Sampling periods were defined according to the periods when the prevalence of Bonamia ostreae and Marteilia sp. (sporulating stage) are highest in the northernmost areas where they have been detected ( Engelsma et al . 2010; A. Alfjorden pers. comm ). The selected sampling sites are shown in Figure 1 and listed in Table 1.
Usually, the surveillance includes an on-site survey, as the state of the population (density, reproduction, signs of mortality) are considered important meta-data. Oysters were collected by skin-diving (Hafrsfjord) or collected from suspended ropes (Innerøyen) and transported to the Institute of Marine Research (IMR) in Bergen. At Askerholmen, mussels were collected by personnel from the Food Safety Authority and sent to IMR Bergen by over-night mail ( Table 1 ). At Langesand, the site was inspected by skin diving. There is no commercial harvest at this site, which is no longer included in the regular surveillance programme. Samples were not collected.
All oysters and mussels were processed at the IMR laboratory in Bergen, according to standard methodology, and under ISO 17025 QA. The samples, consisting of 150 specimens, were split in two, to be analyzed using histology as well as Polymerase Chain Reaction analysis ( PCR)( Table 1 ). Briefly; Histology was performed using dorso-ventral cross sections, fixed in Davidson’s fixative, embedded in paraffin, sectioned at 3 µ m, stained with Hematoxylin Eosin Saffron (HES), mounted with a cover slip and observed at 100 to 1000 x magnification. Samples for PCR were fixed in ethanol. DNA was extracted from ethanol fixed digestive gland tissue. Marteilia detection and typing was done as described by Le Roux et al. (2001).
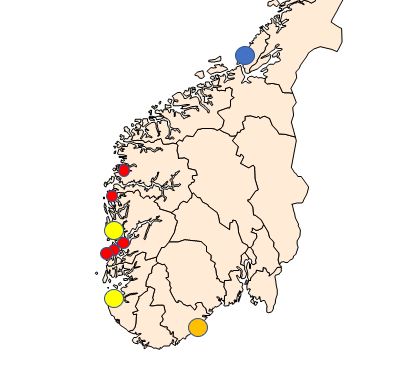
Table 1. Sampling and surveillance of flat oysters ( Ostrea edulis ) and mussels ( Mytilus sp.) in 2021. Samples consist of at least 150 specimens. Each sample is divided in two and analyzed either by histology or PCR. Sites are shown on Figure 1.
| Sampling site | Oysters | Mussels | |||
| Spring-summer | Autumn | Spring | Autumn | ||
| Hafrsfjord, Rogaland | 150 | 104 obs by microscopy, 48 by PCR | |||
| Innerøy, Vestland | 150 | 104 0bs by microscopy 48 by PCR | |||
| Askerholmen, Trøndelag | 150 | 75 obs by microscopy, 77 by PCR | |||
3 - Results
Bonamia spp. was not observed in any sample in 2021. Marteilia sp. was not observed in oysters but M. pararefringens were present in wild mussels from Syljepollen in Bømlo and Hardbakkepollen in Solund municipality (not part of the surveillance programme). Results from the sites listed in Table 1 are briefly described below.
Examination of flat oysters
Langesand ( 58.539225, 8.937646 ). Langesand has a large population of flat oysters. The site was previously included in the surveillance programme and subjected to a targeted Bonamia survey (see previous reports). All samples have been Bonamia -negative. The site has an apparent stable sub-population of flat oysters growing from 3-6 m depth mostly protected from harvesting and climatic events. From 3 m to surface the population has variable recruitment success and survival due to predation, harvesting and ice conditions. Both sub-populations contain specimens of several year-classes with older specimen dominating in the deeper stratum. There was no sign of elevated mortality. Oysters were not sampled for further examination in 2021.
Hafrsfjord ( 58.926864, 5.6436861 ). Hafrsfjord is located west of the city of Stavanger in Rogaland County, Western Norway. The fjord has a narrow inlet and appears as a semi-closed marine fjord-system. Hafrsfjord has a large population of flat oysters. These oysters have been harvested and used in re-stocking projects in the North Sea. Four sites in Hafrsfjord were visited in May. Three of the sites have been harvested to supply the re-stocking programmes. Low to moderate densities and several year-classes of flat oysters were observed. Oysters observed by microscopy appeared normal, had a good condition and variable gonad status. Some shells were infested by Polydora sp. A few intracellular bacterial colonies (RLO) were observed in digestive epithelia in 5 out of 104 oysters.
Innerøy ( 60.1508985, 5.4122928 ). Innerøyen is presently the only site in Norway with a traditional production of flat oyster spat. Oysters were collected from suspended ropes in the mid part of the lagoon in June. The condition index of the oysters was variable, as most oysters were sexually mature, and some had spawned. A few intracellular bacterial colonies (RLO) were observed in digestive epithelia in 5 out of 104 oysters. Haemic neoplasia was observed in one oyster.
Examination of Mussels
Askerholmen, Åfjord, Trøndelag ( 63.9425794, 10.184047 ). Askerholmen is situated near Åfjord in Trøndelag. The site has a commercial mussel farm, owned by the company Norgeskjell. The farm is close to the dispatch center and is considered a point of interception for mussels harvested by Norgeskjell and packed there. Seventy-five mussels were examined by microscopy. Mussels appeared in good but variable condition. Haemic neoplasia was observed in one mussel. No other abnormal finding was recorded.
4 - Discussion and conclusions
The health status of flat oysters
Norwegian flat oysters, O. edulis , appear free from Bonamia spp. Healthy flat oysters is a valuable resource. It is important to monitor Norwegian stocks and disseminate the information on their health status to obtain a consensus on how to protect and care for this resource. We are monitoring Norwegian flat oyster populations and aim at using the data gained in a Nordic and European context. The monitoring of stocks is linked to the national health surveillance, and through the contact with European scientists to both genetic studies ( Margen project) and re-stocking programmes ( https://noraeurope.eu/ ). Flat oysters from Hafrsfjord in Rogaland have already been translocated and seeded on hard bottom sites off the coast of The Netherlands. The wild flat oysters from Hafrsfjord examined in 2021 appear healthy, with a normal reproductive cycle pattern. Intracellular Rickettsia-like colonies and haemic neoplasia were observed, but at low prevalence and intensity. These are common observations, and not considered a problem, although the neoplasia may develop into disease problems and potentially induce winter mortalities of affected flat oysters as their nutrient uptake and immune defense are knocked out ( Mortensen et al . 2013 ).
Examination of mussels
In Norway, Marteilia pararefringens has been found in blue mussels, Mytilus sp, in four traditional heliothermic marine oyster lagoons, two wild populations and one mussel farm in western Norway (see Figure 1 and report from 2020 ( Mortensen & Skår 2021 )). These findings indicate that M. pararefringens is not restricted to the heliothermic lagoons. We have started a survey including several of the traditional oyster lagoons as well as mussels in closed, sheltered areas. To optimize the sampling regime in the surveillance programme and the Marteilia study, we collaborate with all active mussel producers to map the farms, spat collection areas and translocations. Most of the Norwegian mussel production is located in Trøndelag and Nordland. All farmed mussels from this (large) area are processed, packed and distributed by two dispatch centers, owned by Norgeskjell. Although two selected sites (close to the dispatch centres) are included in the surveillance programme, we do not have a proper overview of the production in this region. The dispatch centers will be followed up and their sources of mussels mapped. It is important to establish a surveillance model for this area and initiate a targeted sampling . Marteilia refringens has never been detected in Norwegian flat oysters. Based on our findings, M. pararefringens does not seem to infect flat oysters. It is however important to be sure that oysters do not act as vectors. We will thus continue to combine surveillance and research activity to obtain as much data as possible, also from oysters at the infected site, and from more sites that have been in contact with the former network of oyster producers. A targeted study of oysters at the infected site at Bømlo will be carried out in 2022.
Progress of the surveillance
The surveillance will be continued according to the plan discussed with the Norwegian Food Safety Authority, and with the aim of obtaining a time series of data on the health status of flat oysters and mussels along the Norwegian coast. The surveillance programme will be focused on mussel and oyster farms and/or populations that are commercially harvested for on-growth, transport to the markets or used in re-stocking programmes. The detection of Marteilia pararefringens in mussels in new areas indicate that this parasite is more widely distributed than previously known. The geographical survey will be continued during summer 2022. A model based on one production area / zone in Trøndelag / Nordland will be proposed.
The health surveillance is linked to monitoring of stocks, oyster genetic studies and European oyster projects, strengthening the scientific basis for a strong and adequate management of the few remaining, healthy flat oyster populations in Europe. It is also important to avoid introduction of Pacific oysters into sites that are used as sources of disease-free flat oyster seed for aquaculture purposes.
Flat oysters do not appear susceptible to M. pararefringens. Scientific studies focus on understanding the infection and life cycle of M. pararefringens in mussels, as a background for a risk assessment and an epidemiological study of the spreading of this parasite in North European mussel populations.
Marteilia refringens Type O has never been detected in Norwegian mollusks. To maintain the disease-free status for Marteilia refringens in oysters, a targeted sampling of flat oysters will be carried out at Bømlo (where mussels are infected with M. pararefringens ) as well as in a commercially exploited population in Agder, Southern Norway.
We consider Norwegian populations of flat oysters as free from Bonamia spp. We advise the Norwegian Food Safety Authority to apply for disease free status for Bonamia ostreae and B. exitiosa in Norwegian flat oysters. If additional samples are required to fulfil the criteria, this should be included in the sampling plan before May.
Acknowledgements
Thanks to Ingrid U. Fiksdal and Kai Skaftnesmo for technical assistance.
5 - References
Commission Delegated Regulation (EU) 2020/689 of 17 December 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for surveillance, eradication programmes, and disease-free status for certain listed and emerging diseases. http://data.europa.eu/eli/reg_del/2020/689/oj
Engelsma MY, Kerhoff S, Roozenburg I, Haenen OLM, van Gool A, Sistermans W, Wijnhoven S, Hummel H (2010). Epidemiology of Bonamia ostreae infecting European flat oysters Ostrea edulis from Lake Grevelingen, The Netherlands. Marine Ecology Progress Series 409: 131 – 142.
Kerr R, Ward GM, Stentiford GD, Alfjorden A, Mortensen S, Bignell JP, Feist SW, Villalba A, Carballal MJ, Cao A, Arzul I, Ryder D, Bass D (2018). Marteilia refringens and Marteilia pararefringens sp. nov. are distinct parasites of bivalves and have different European distributions. Parasitology 1–10. https://doi.org/10.1017/ S003118201800063X
Le Roux F, Lorenzo G, Peyret P, Audemard C, Figueras A, Vivares C, Gouy M, Berthe F (2001). Molecular evidence for the existence of two species of Marteilia in Europe. Journal of Eukaryote Microbiology 48: 449-454.
Mortensen S, Skår C (2020). The surveillance and control programme for bonamiosis and marteiliosis in European flat oysters, Ostrea edulis , and blue mussels, Mytilus sp. in Norway in 2019. Rapport fra havforskningen nr 36, 2020, 16 s. https://www.hi.no//hi/nettrapporter/rapport-fra-havforskningen-en-2020-36
Mortensen S, Skår CK, Harkestad LS (2013). Hemisk neoplasi hos norsk flatøsters, Ostrea edulis . Norsk Veterinærtidsskrift 125 (7): 438 -442.
Mortensen S, Sælemyr L, Skår CK, Bodvin T, Jelmert A (2016). Health surveillance of the flat oyster populations in Aust-Agder County, southern Norway in the period 2009 – 2015. Rapport fra havforskningen nr 11, 2016, 11s.
Mortensen S, Skår C, Sælemyr L (2020). Summarizing the screening for Bonamia ostreae in Norwegian populations of flat oysters, Ostrea edulis . Rapport fra Havforskningen no 24, 2020. ISSN:1893-4536, https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-en-2020-24
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). https://eur-lex.europa.eu/eli/reg/2016/429/oj
Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products. http://data.europa.eu/eli/reg/2017/625/oj
Sas H, Deden B, Kamermans P, zu Ermgassen P S E, Pogoda B, Preston J, Helmer L, Holbrook Z, Arzul I, van der Have T, Villalba A, Colsoul B, Lown A, Merk V, Zwerschke N, Reuchlin E (2020). Bonamia infection in native oysters ( Ostrea edulis ) in relation to European restoration projects. Aquatic Conservation. Marine and Freshwater Ecosystems 30: 2150-2162. https://doi.org/10.1002/aqc.3430
