Overvåkingsprogrammet for sykdommene bonamiose og marteiliose i flatøsters utføres av Havforskningsinstituttet på oppdrag fra Mattilsynet. Det ble i 2023 hentet flatøsters fra fire lokaliteter: en poll hvor det produseres østersyngel, en brakklagt poll hvor det tidligere ble produsert østersyngel og to fjordsystemer med større forekomster av flatøsters. Prøvene ble samlet inn i april-juni og september, som er de periodene prevalensen av parasittene Bonamia spp. og Marteilia spp. er vist å være høyest i smittede bestander. Det ble ikke observert unormal dødelighet. Bonamia ostreae / B. exitiosa ble ikke påvist. Marteilia refringens ble ikke påvist. Marteilia refringens Type O og M er nå splittet i to arter; Marteilia refringens i flatøsters og M. pararefringens i blåskjell. Blåskjell er derfor ikke lenger regnet som mottakelig vert for M. refringens, men vårt forskningsarbeid viser at den beslektede Marteilia pararefringens er utbredt i enkelte bestander langs Sør- og Vestlandet. M. pararefringens kan ha alvorlig effekt på blåskjellbestandene og det bør vurderes om parasitten skal listeføres på nasjonal liste. Videre foreslås det om å søke om etablering av fristatus for Bonamia spp. og Marteilia refringens i norsk flatøsters.
The surveillance and control program for bonamiosis and marteiliosis in European flat oysters, Ostrea edulis in 2023
Rapportserie:
Rapport fra havforskningen 2024-14
ISSN: 1893-4536
Overvåkingsgruppens rapporter
Publisert: 25.04.2024
Oppdatert: 24.11.2025
Prosjektnr: 14538
Oppdragsgiver(e): Mattilsynet
Referanse: Odd Arne Brimsøe
Forskningsgruppe(r):
Smittespredning og sykdom
,
Bunnsamfunn
Tema:
Flatøsters,
Blåskjell
Program:
Miljøeffekter av akvakultur,
Kystøkosystemer
Approved by:
Research Director(s):
Geir Lasse Taranger
Program leader(s):
Jan Atle Knutsen og Mari Skuggedal Myksvoll
English summary
Sammendrag
The surveillance program for the molluscan diseases bonamiosis and marteiliosis is carried out by the Institute of Marine Research according to a contract with the Norwegian Food Safety Authority. In 2023, flat oysters were sampled from four locations: A breed poll where oyster spat is produced, an abandoned breed poll where oyster spat was previously produced, and two fjord systems with larger populations of flat oysters. Samples were collected in April – June and September, to be able to detect Bonamia sp. and Marteilia sp. during the periods when the potential prevalence is highest. No abnormal mortalities were observed during the surveillance. Bonamia ostreae / B. exitiosa and Marteilia refringens were not detected. Marteilia refringens Type O and M are now divided into two species; Marteilia refringens in flat oysters and M. pararefringens in blue mussels. Blue mussels are therefore no longer considered susceptible hosts for M. refringens, but our research project shows that the related Marteilia pararefringens is widespread in certain mussel populations along the South and West coasts of Norway. M. pararefringens can have a serious impact on blue mussel populations, and consideration should be given to listing the parasite on a national list. Furthermore, we propose a formal application for disease free status for Bonamia spp. and Marteilia refringens in Norwegian flat oysters
1 - Introduction
Production and harvest of mollusks in Norway
Norway has a small shellfish industry, which is relatively stable, with approximately 60 mussel farms, a few flat oyster farms and four dispatch centres distributing farmed as well as wild caught shellfish. In 2023, the commercial production (from aquaculture + harvest) was approximately 1 200 tons of mussels, Mytilus spp., 8 tons of flat oysters, Ostrea edulis, and 30 tons of wild Pacific oysters, Magallana (Crassostrea) gigas. Around 400 tons of scallops, Pecten maximus, were harvested from wild beds. A fishery for Iceland scallops, Chlamys islandica, was re-established and the harvest in 2023 was 7 469 tons. There was also a small harvest of clams and horse mussels collected by divers. One oyster lagoon (Innerøyen) produced flat oyster seed, but the production was kept for on-growth at the site. Nothing was sold in 2023. There was no export. 250 tons of blue mussels were imported from Denmark and Pacific oysters were imported from France (un-known volume). All import were placed directly on the market.
Monitoring of wild oyster populations
The Institute of Marine Research (IMR) carries out a surveillance of the wild oyster stocks to describe the dynamics of the oyster populations and detect changes – including mortality events and establishment of the invasive Pacific oyster, Magallana (Crassostrea) gigas in mussel and flat oyster habitats. Reports from the surveillance is proposed as a basis for management and to obtain a sustainable harvest. The monitoring of populations is linked to the disease surveillance programme.
The status of bonamiosis and marteiliosis in European flat oysters, Ostrea edulis, and blue mussels, Mytilus sp. in Norway
The health status of Norwegian flat oysters has been studied since 1989. A surveillance program for bonamiosis and marteiliosis in flat oysters was initiated in 1995. Mussels were included in 2010. Since 2015, the surveillance program has been carried out by the Institute of Marine Research according to a contract with the Norwegian Food Safety Authority. The over-all aim is to gain knowledge on the health situation of farmed and commercially exploited Norwegian flat oysters and mussels.
Bonamia ostreae, Bonamia exitiosa and Marteilia refringens have never been detected in flat oysters during the surveillance. After observation of microcells in haemocytes in flat oysters from Langesand in Agder County, southern Norway in 2008, samples were sent from the National Veterinary Institute to EURL. Samples from two oysters tested PCR-positive for B. ostreae. A containment zone was established, and a targeted surveillance was initiated and followed up with annual sampling. An almost continuous sampling and analysis of several thousand oysters since 2009 has never confirmed Bonamia at this site. Although the microcells may be observed in the oysters, they only appear as single cells, with low intensity and low prevalence in the population. There is no sign of pathogen propagation, no pathology associated with the observed cells and no abnormal mortality in the population (see previous reports and Mortensen et al. 2020). Based on the results we have obtained so far; we conclude that the observed microcells are not B. ostreae.
The entire coastline of Norway is a disease-free zone with regard to B. ostreae with the exception of the zone in Agder. We have initiated an increased surveillance with a three-year annual sampling of 150 oysters every spring and a bi-annual sampling of a commercially exploited flat oyster population in the same region to fulfil the criteria for the re-establishment of disease-free status in compliance with EU 2020/689. However, Langesand has been extensively sampled since 2009, with 100 – 150 oysters sampled per year since 2015, with the exception of the period 2018 to 2022 (see Mortensen et al. 2016 and Appendix).
The entire coastline of Norway is a disease-free zone also with regard to Marteilia refringens, with the exception of the containment area in the municipality of Bømlo in Vestland County, southern Norway. The containment area was established in 2017 due to detection of M. refringens Type M in blue mussels. This type is now classified as a distinct species, Marteilia pararefringens, infecting mussels only (Kerr et al. 2018). M. refringens is regarded as specific for flat oysters. The Norwegian Food Safety Authority will put forward a proposal to lift the restrictions at Bømlo, because blue mussels are not listed as susceptible species for M. refringens. Due to the de-listing of mussels as susceptible species for M. refringens , mussels were not sampled in 2023. M. pararefringens is present in Norwegian blue mussels.
This report gives a brief overview of the present situation, results from 2023 and the prospects for the work in 2024.
2 - Material and methods
Surveillance plans have been designed for maintaining the disease-free statuses for Bonamia spp. and Marteilia refringens, as defined in Regulation (EU) 2016/429 and Delegated Regulation (EU) 2020/689.
Sample size and frequency (time of year) has been changed during the surveillance period. Bi-annual sampling of 150 specimens in two of the targeted populations (Hafrsfjord and Innerøyen) was initiated in 2021. Annual sampling of 150 specimens over a three-year period in the containment area in Agder was conducted between 2015 and 2017 and started again in 2023 (see Appendix).
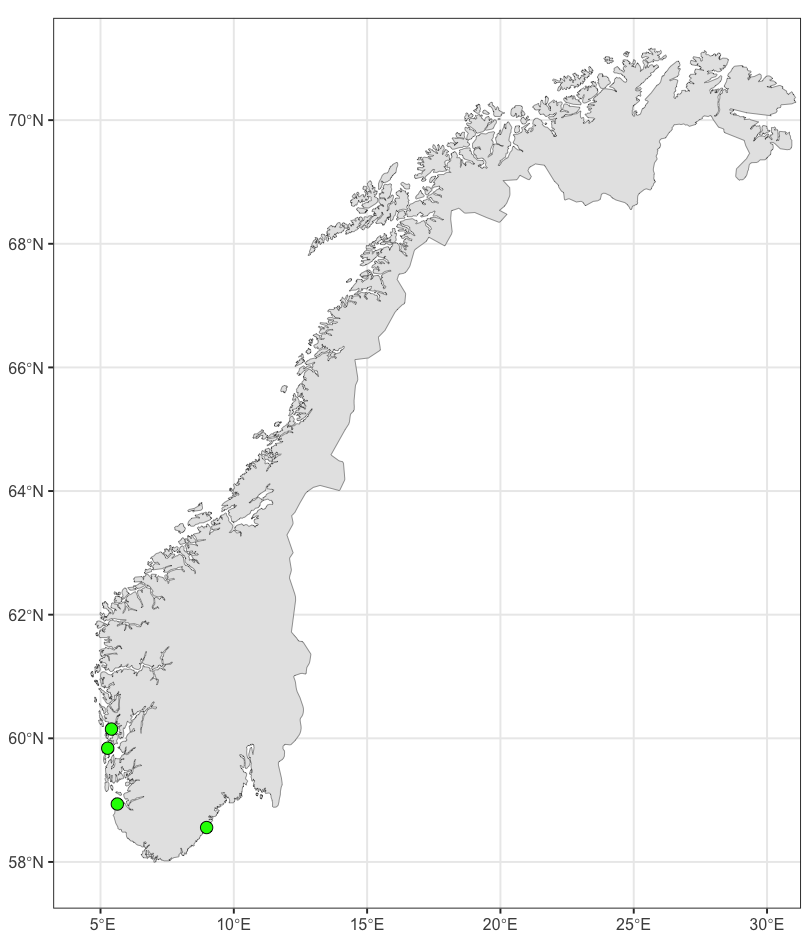
Sampling period was defined according to the period when the prevalence of Bonamia ostreae is highest in the northernmost areas where they have been detected ( Engelsma et al. 2010). The selected sampling sites are shown in Figure 1 and listed in Table 1. Usually, surveillance includes an on-site survey, as the state of the population (density, reproduction, signs of mortality) are considered important meta-data. Collection of oysters was done by swimmers/divers (NRL staff) and transported cold and humid directly to laboratory and kept in quarantine lab until dissection.
In 2023, oysters were sampled from one wild oyster population in Hafrsfjord, Rogaland (west coast) which is used as a source of oysters for hatchery broodstock as well as restocking projects, one wild oyster population in Langesand, Agder, one farmed population at Innerøyen, Vestland (an active breed- and spat-poll) and one in an abandoned breed poll at Aga, Vestland (see Figure 1 and Table 1).
Oysters were processed at the IMR laboratory in Bergen, according to standard methodology. Histology aiming at the detection of Bonamia spp. and Marteilia spp. was carried out according to NS-EN ISO / IEC 17025.
The samples, consisting of 150 or 152 specimens, were split in two, to be analyzed using histology as well as Polymerase Chain Reaction analysis (PCR). Samples from Innerøyen were analyzed by PCR only (Table 1). Histology was performed using dorso-ventral cross sections, fixed in Davidson’s fixative, embedded in paraffin, sectioned at 3 µ m, stained with Hematoxylin Erythrosin Saffron (HES), mounted with a cover slip and observed at 100 to 1000 x magnification. Samples for PCR were fixed in ethanol. DNA was extracted from ethanol fixed digestive gland tissue for Marteilia detection and typing was done by PCR as described by Le Roux et al. (2001). Samples for Bonamia detection were sampled from gill tissue and analyzed as described by Cochennec et al. (2020) .
| Sampling site | Sampling date | Sample size | Method |
|---|---|---|---|
| Agapollen, Vestland | 9. Sept. | 218 | All by PCR and microscopy. Only Marteilia PCR. |
| Innerøyen, Vestland | 24. April | 152 | All by Bonamia PCR |
| Hafrsfjord, Rogaland | 23. June | 150 | 75 obs by microscopy, 75 by Bonamia PCR |
| Langesand, Agder | 12. May | 152 | 75 obs by microscopy, 77 by Bonamia PCR |
3 - Results
No abnormal mortalities were observed during the surveillance. Bonamia ostreae / B. exitiosa and Marteilia refringens were not detected. Results from the sites listed in Table 1 are briefly described below.
Sampling and examination of flat oysters Langesand ( 58.5392, 8.9376 )
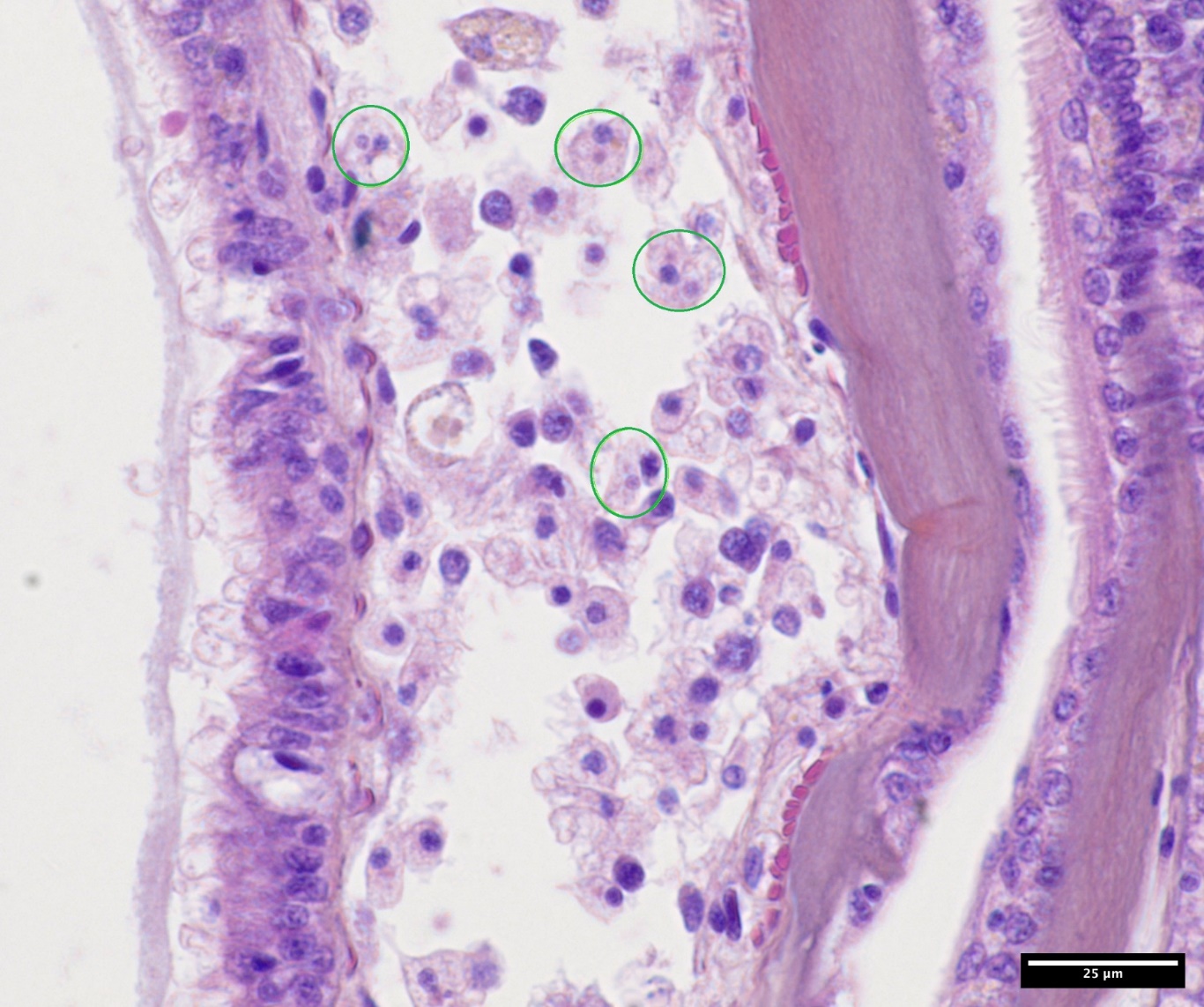
Langesand and the surrounding area has a large population of flat oysters. The site was previously included in the surveillance program and subjected to a targeted Bonamia survey (see Mortensen et al. 2020 and previous reports). The site has an apparent stable sub-population of flat oysters growing from 3-6 m depth mostly protected from harvesting and climatic events. From 3 m to the surface the population has variable recruitment success and survival due to predation, harvesting and ice conditions. Both sub-populations contain specimens of several year-classes with older specimens dominating in the deeper stratum. There was no sign of elevated mortality. The oysters appeared in good condition and from immature to maturing gonads. Very few, single microcells were observed in haemocytes in gill and connective tissues in seven oysters, without any sign of inflammation or pathological alterations. Early stage of haemic neoplasia was observed in one oyster (see Mortensen et al. 2013).
Hafrsfjord (58.9262, 5.6472)
Hafrsfjord is a large, sheltered fjord system with a shallow and relatively narrow opening to the outside open water. The fjord has large shallow areas functioning as oyster habitats. Water temperature during summer is high enough for maturation and spawning. A field study performed in June 2023 revealed flat oysters inhabiting most of the shallow water areas, down to 4-5 m depth and with a variable density, in some areas patchy, with up to 10 oysters per square meter (Mortensen et al. 2023). Several year classes were present. There was no sign of mortality. The oysters were in good condition, with high energy reserves, mature gonads and entering the spawning period. Early neoplasia was observed in one oyster. A potential microcell was observed in a haemocyte in gill tissue in one oyster.
Innerøyen (60.1507, 5.4132)
Innerøyen is the last oyster poll in operation in Norway. The poll has a local, self – sustaining population of flat oysters. Oyster seed is collected on different types of spat collectors. Oysters and mussels from this site have been sampled since the initiation of the surveillance program and inspected annually. The condition of the oysters was variable, indicating food limitation in parts of the poll. All tissues appeared normal during dissection. Oysters were analyzed by Bonamia PCR only.
Aga (59.8399, 5.2475)
Agapollen is a former production site which produced oyster seed during most of the period between 1882 and 2010. There is no oyster production today, but there are still wild oysters present in the poll. Agapollen is where we detected Marteilia pararefringens in mussels in 2016. In 2023 we sampled local flat oysters that were collected in the poll and flat oysters originating from Innerøypollen, deployed in Aga as part of a field experiment in 2022:
A total of 114 local flat oysters were analyzed by histology and screened for bonamiosis and marteiliosis by microscopy; neither Bonamia nor Marteilia was detected in any of the samples. Disseminated neoplasia was observed in six oyster samples, and seven samples had moderate hemocyte infiltration in the vesicular connective tissue and gonadal tissue. Gonadal status was also assessed and described as males (n=36), females (n=35), hermaphrodite (n=12) or spent (n=31). Gonad degeneration, mostly in females, hemocyte infiltration in the follicles, and dead sperm cells were a common observation in several samples.
A total of 104 flat oysters originating from Innerøypollen were analyzed by histology and screened for bonamiosis and marteiliosis by microscopy; neither Bonamia nor Marteilia was detected in any of the samples. However, microcells, similar to those found in flat oysters from Langesand, were observed in three individuals. Disseminated neoplasia was observed in five oysters.
4 - Discussion and conclusions
The health status of Norwegian flat oysters
Marteilia refringens has never been detected in Norway, and Norwegian flat oysters, Ostrea edulis, appear free from Bonamia spp.
Based on the results we have obtained so far; we conclude that the observed microcells are not Bonamia ostreae. Though they appear non-pathogenic, further research will be required to elucidate their relationship to Bonamia or similar microcell parasites. Healthy flat oysters are a valuable resource. It is important to monitor Norwegian stocks and disseminate the information on their health status to obtain a consensus on how to protect and care for this resource. We are monitoring Norwegian flat oyster populations and aim at using the data gained in a Nordic and European context. The monitoring of stocks is linked to national health surveillance, and through the contact with European scientists to both genetic studies (Margen project) and re-stocking programs (https://noraeurope.eu/).
There is a growing interest in the re-establishment of wild oyster beds in Northern Europe. The restoration is dependent on the availability of flat oysters free from Bonamia spp. and M. refringens (Sas et al. 2020). Both the oyster farming industry and re-stocking projects therefore focus on where to find naïve flat oyster populations that are free from Bonamia spp., as well as other pathogens that may affect the populations. In the present situation – and after the re-occurrence of B. ostreae in Limfjorden, Denmark, in 2014 (Madsen & Thomassen 2015) – safe sources of oysters can only be found in Sweden and Norway. Norwegian populations of European flat oysters have been monitored since 1989 and are considered free from notifiable diseases (Mortensen 1989; Mortensen et al. 2016; 2020). Oysters from Hafrsfjord in Rogaland are being used in re-stocking trials in the North Sea. Oysters from this area are included in the surveillance program, and the stocks in Hafrsfjord are monitored every 2 years.
There is a small commercial harvest of wild flat oysters along the south and south-western coast of Norway. During this harvest, half-grown oysters are sometimes collected and used as seed in oyster farms. One example is Sørskjell Ltd, located in Arnevik, which was included in the surveillance in 2022. Here, the shellfish farmer produces both mussels and oysters in suspended culture and uses local beds of oysters both as a bottom culture and a source of half-grown oysters for the farm. The production is efficient but demonstrates the link between wild and farmed oysters
Marteilia infections in mussels
Though mussels were not sampled in 2023 since they are not susceptible hosts for M. refringens, M. pararefringens is still present in several populations. Bøgwald & Mortensen (in press) have shown that the parasite can cause severe infections across its range and has a much greater (though restricted) distribution than previously thought. This pathogen may thus be included in a national list of notifiable diseases. IMR is carrying out further studies to clarify its habitat and host requirements as a background for management.
Conclusions and recommendations
The surveillance will be continued according to the plan discussed with the Norwegian Food Safety Authority, and with the aim of obtaining a time series of data on the health status of flat oysters along the Norwegian coast. The surveillance programme will be focused on oyster farms and/or populations that are commercially harvested for on-growth, transport to the markets or used in re-stocking programmes.
All historical data show that Marteilia refringens, Bonamia ostreae and B. exitiosa are absent in Norwegian flat oysters. No abnormal mortalities have been recorded and all sites are considered low-risk areas, and the general approach is to cover the main production sites with two-year intervals and one sample per year, collected in spring – early summer. Sampling is combined with surveillance of stock, that will reveal abnormal mortality or decrease of stocks. In the present surveillance, one sample covers both Marteilia refringens, Bonamia ostreae and B. exitiosa. It needs to be clarified if maintenance of freedom status requires an additional sampling in late summer or autumn.
Mussels were not sampled in 2023. As mussels are not regarded a susceptible host for M. refringens, and M. pararefringens is not listed, it remains to be clarified whether mussels shall be included in the surveillance. M. pararefringens is pathogenic to mussels, may be diagnosed and are restricted to certain sites. This pathogen may thus be included in a national list of notifiable diseases.
To maintain the disease-free status for M. refringens in oysters, a targeted sampling of flat oysters will be continued at Bømlo (where mussels are infected with M. pararefringens), Langesand, as well as in a commercially exploited population in Agder, Southern Norway in 2024.
We consider Norwegian populations of flat oysters as free from Bonamia spp. We advise the Norwegian Food Safety Authority to apply for disease free status for B. ostreae and B. exitiosa in Norwegian flat oysters when the requirements for a three-year surveillance period in Agder is fulfilled in 2025.
Health surveillance is linked to monitoring of stocks, oyster genetic studies and European oyster projects, strengthening the scientific basis for a strong and adequate management of the few remaining, healthy flat oyster populations in Europe. It is also important to avoid introduction of Pacific oysters into sites that are used as sources of disease-free flat oyster seed for aquaculture purposes.
A management plan for Norwegian flat oysters will be proposed in 2025, with a special focus on how to protect some of the dense wild oyster populations in Southern Norway.
Acknowledgements
Thanks to Lars-Johan Naustvoll for sampling at Langesand, Tore Strohmeier and Øivind Strand for assistance during field work and sampling in Rogaland and Julie M. Aga and Kai Skaftnesmo for technical assistance.
5 - References
Bøgwald M, Mortensen S (in press). Infections with Marteilia pararefringens in wild mussels, Mytilus spp., along the Norwegian coast are more frequent than revealed by the national surveillance programme and highlight the need for its improvement. Diseases of Aquatic Organisms. https://doi.org/10.3354/dao03785
Cochennec, N., Le Roux, F., Berthe, F., Gerard, A. (2000). Detection of Bonamia ostreae based on small subunit ribosomal probe. Journal of Invertebrate Pathology 76: 26-32.
Commission Delegated Regulation (EU) 2020/689 of 17 December 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for surveillance, eradication programmes, and disease-free status for certain listed and emerging diseases. http://data.europa.eu/eli/reg_del/2020/689/oj
Engelsma MY, Kerhoff S, Roozenburg I, Haenen OLM, van Gool A, Sistermans W, Wijnhoven S, Hummel H (2010). Epidemiology of Bonamia ostreae infecting European flat oysters Ostrea edulis from Lake Grevelingen, The Netherlands. Marine Ecology Progress Series 409: 131 – 142.
Kerr R, Ward GM, Stentiford GD, Alfjorden A, Mortensen S, Bignell JP, Feist SW, Villalba A, Carballal MJ, Cao A, Arzul I, Ryder D, Bass D (2018). Marteilia refringens and Marteilia pararefringens sp. nov. are distinct parasites of bivalves and have different European distributions. Parasitology 1–10. https://doi.org/10.1017/S003118201800063X
Le Roux F, Lorenzo G, Peyret P, Audemard C, Figueras A, Vivares C, Gouy M, Berthe F (2001). Molecular evidence for the existence of two species of Marteilia in Europe. Journal of Eukaryote Microbiology 48: 449-454.
Madsen L, Thomassen HEH (2015). First detection of Bonamia ostreae in native flat oysters from the Limfjord in Denmark. In: European Association of Fish Pathologists, International Conference on Diseases of Fish and Shellfish: Abstract book (pp 92). (O-084), Las Palmas.
Mortensen SH (1993). A health survey of selected stocks of commercially exploited Norwegian bivalve molluscs. Diseases of Aquatic Organisms 16: 149-156. Mortensen S.
Bøgwald M, Strohmeier T, Strand Ø, Larsson SB, Laugen AT, Reamon MC, Marcussen JB (2023). Kartlegging av østersbestander i Rogaland i 2021 og 2023 - innsamling av data som grunnlag for bestandsforvaltning. Rapport fra havforskningen 2023-55 ISSN: 1893-4536. 22 s.
Mortensen S, Skår CK, Harkestad LS (2013). Hemisk neoplasi hos norsk flatøsters, Ostrea edulis . Norsk Veterinærtidsskrift 125 (7): 438 -442.
Mortensen S, Sælemyr L, Skår CK, Bodvin T, Jelmert A (2016). Health surveillance of the flat oyster populations in Aust-Agder County, southern Norway in the period 2009 – 2015. Rapport fra havforskningen nr 11, 2016, 11s.
Mortensen S, Skår C (2020). The surveillance and control programme for bonamiosis and marteiliosis in European flat oysters, Ostrea edulis , and blue mussels, Mytilus sp. in Norway in 2019. Rapport fra havforskningen nr 36, 2020, 16 s. https://www.hi.no//hi/nettrapporter/rapport-fra-havforskningen-en-2020-36
Mortensen S, Skår C, Sælemyr L (2020). Summarizing the screening for Bonamia ostreae in Norwegian populations of flat oysters, Ostrea edulis. Rapport fra Havforskningen no 24, 2020. ISSN:1893-4536, https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-en-2020-24
Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). https://eur-lex.europa.eu/eli/reg/2016/429/oj
Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products. http://data.europa.eu/eli/reg/2017/625/oj
Sas H, Deden B, Kamermans P, zu Ermgassen P S E, Pogoda B, Preston J, Helmer L, Holbrook Z, Arzul I, van der Have T, Villalba A, Colsoul B, Lown A, Merk V, Zwerschke N, Reuchlin E (2020). Bonamia infection in native oysters ( Ostrea edulis ) in relation to European restoration projects. Aquatic Conservation. Marine and Freshwater Ecosystems 30: 2150-2162. https://doi.org/10.1002/aqc.3430
Appendix
| Number of sampled flat oysters | |||
|---|---|---|---|
| Year | Sample site | Spring | Autumn |
| 2015 | Hui | 30 | 30 |
| Langesand | 150 | 150 | |
| Hafrsfjord | 30 | 30 | |
| Sveio | 30 | 30 | |
| 2016 | Langesand | 55 | |
| Hafrsfjord | 30 | 30 | |
| Sveio | 30 | ||
| Aga | 30 | 30 | |
| 2017 | Langesand | 55 | |
| Hafrsfjord | 30 | 30 | |
| Sveio | 30 | 30 | |
| Aga | 30 | 30 | |
| 2018 | Langesand | 55 | |
| Hafrsfjord | 30 | 30 | |
| Sveio | 30 | ||
| Aga | 30 | 30 | |
| 2019 | Langesand | 62 | |
| Aga | 30 | ||
| Innerøyen | 30 | 30 | |
| 2020 | Hafrsfjord | 100 | |
| Innerøyen | 30 | ||
| Haugavågen | 99 | ||
| Vågstranda | 18 | ||
| Ostrevig | 30 | ||
| 2021 | Hafrsfjord | 150 | |
| Innerøyen | 150 | ||
| 2022 | Arnevik | 152 | |
| 2023 | Langesand | 152 | |
| Hafrsfjord | 150 | ||
| Aga | 218 | ||
| Innerøyen | 152 | ||
