This report describes the results from The surveillance and control programme for bonamiosis and marteiliosis and research activities on Marteilia sp. in 2019. The programme is carried out by the Institute of Marine Research according to a contract with the Norwegian Food Safety Authority. Samples were collected from two oyster farms, one wild oyster population, two mussel farms and four wild beds, two mussel farms and five wild mussel populations. Samples were collected in April/May and in October, in order to be able to detect Bonamia sp. and Marteilia sp. during the periods when the potential prevalence is at the highest. Samples from one of the mussel farms and one wild bed were collected after reports of elevated mortality. Except from these samples, no abnormal mortalities were observed during the surveillance. Bonamia ostreae / B. exitiosa were not detected. There have been several reports on mortality or “disappearance” of mussels along the Norwegian coast. The reason(s) for the mortalities have not been determined. However, the parasite Marteilia sp. was detected for the first time in mussels, Mytilus edulis, collected at Bømlo, western Norway in 2016 and Tysnes in 2019. This has been followed up with an extended survey in several research projects. The results show that Marteilia sp. infecting mussels in Norway, Sweden and England are different from Marteilia refringens infecting flat oysters. The name Marteilia pararefringens has been proposed, and there is strong evidence that Marteilia refringens and Marteilia pararefringens sp. nov. are distinct parasites of bivalves and have different European distributions. The work will be continued in 2020 – 2021, linked to research on the distribution of M. pararefringens in wild mussels. We propose a revision of the surveillance programme combined with the establishment of a new model for health control in mollusk farms, application for disease free status for Norwegian flat oysters and a categorization of zones.
The surveillance and control programme for bonamiosis and marteiliosis in European flat oysters, Ostrea edulis, and blue mussels, Mytilus sp. in Norway in 2019
Rapportserie:
Rapport fra havforskningen 2020-36
ISSN: 1893-4536
Publisert: 16.10.2020
Prosjektnr: 14538
Oppdragsgiver(e): Mattilsynet
Referanse: Jonathan Vaz Serrano
Forskningsgruppe(r):
Smittespredning og sykdom
Tema:
Blåskjell,
Flatøsters
Program:
Miljøeffekter av akvakultur
Godkjent av:
Forskningsdirektør(er):
Geir Lasse Taranger
Programleder(e):
Terje Svåsand
English summary
Sammendrag
Denne rapporten beskriver Overvåkingsprogrammet for sykdommene bonamiose og marteiliose i flatøsters og blåskjell, og forskningsaktivitet knyttet til funn av Marteilia sp. i 2019. Overvåkingsprogrammet utføres av Havforskningsinstituttet på oppdrag fra Mattilsynet. Det ble hentet skjell fra to dyrkingsanlegg for østers og en vill østersbestand, tre blåskjellanlegg og fem ville blåskjellbestander. Prøvene ble samlet inn i april/mai og i oktober, når prevalensen av parasittene Bonamia spp. og Marteilia spp. er vist å være høyest i smittede bestander. Prøvene fra ett av blåskjellanleggene og en vill bestand (Haugevågen) ble samlet inn etter rapporter om forhøyet dødelighet. Bortsett fra disse ble det ikke observert unormal dødelighet verken vår eller høst. Bonamia ostreae / B. exitiosa ble ikke påvist. Det er kommet inn en rekke rapporter om at blåskjell «forsvinner» mange steder langs kysten. Årsakene til dette er ikke kjent. Parasitten Marteilia sp. ble imidlertid for første gang påvist i ville blåskjell, Mytilus edulis, på Bømlo i 2016, og på Tysnes i 2019. Påvisningen er fulgt opp med en utvidet prøvetaking i flere forskningsprosjekter. Resultatene viser at Marteilia sp. fra blåskjell i Norge, Sverige og England er ulik Marteilia refringens som forårsaker sykdom hos flatøsters og er foreslått gitt navnet Marteilia pararefringens. Det ser således ut til at Marteilia refringens og Marteilia pararefringens sp. nov. er ulike arter med ulike vertsarter (hhv østers og blåskjell). Resultatene fra overvåkingen vil bli fulgt opp med en utvidet prøvetaking av ville blåskjellbestander i 2020 – 2021 i regi av forskningsprosjektene. Det foreslås en revisjon av overvåkingsprogrammet i sammenheng med etablering av en modell for helseovervåking av skjellanlegg, søknad om etablering av fristatus for Bonamia spp. i norsk flatøsters og en gjennomgang av kategoriseringen av soner.
1 - Introduction
The health status of the European flat oyster, Ostrea edulis
The European flat oyster stocks are in a state of crisis. Wild populations have been severely affected by diseases, extensive harvesting and destruction of habitats. The single, most severe factor affecting this species is the introduction of the parasite Bonamia ostreae in 1979 (Elston et al. 1986), and more recently B. exitiosa (Abollo et al. 2008). These parasites have been spread over the most of Europe with translocations of live oysters. The effects are devastating. In most oyster producing areas, aquaculture of flat oysters is no longer possible due to high mortalities caused by bonamiosis.
There is a growing interest in the re-establishment of wild oyster beds in Northern Europe, and initiatives united in the NORA project (https://noraeurope.eu/) now work to make develop a protocol on how to select, treat and seed oysters on some of the earlier oyster beds. We are collaborating with this project, both by contributing to their biosecurity manual and linking the selection of potential oyster source population to the on-growing health monitoring and research.
Oysters affected by bonamiosis may of course not be used in re-stocking projects, and there is therefore an increased focus on where to find naïve flat oyster populations that are free from Bonamia spp, as well as other pathogens that may affect the populations. In the present situation – and after the re-occurrence of Bonamia ostreae in Limfjorden, Denmark, in 2014 (https://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=17298) – safe sources of oysters can probably only be found in Sweden and Norway.
Norwegian populations of European flat oysters have been followed since 1989 and are considered free from notifiable diseases, based on historical data, as well as results from health surveillance. In 2006, microcells resembling the oyster parasite Bonamia sp. were observed during histopathological examination of tissue specimens of flat oysters, Ostrea edulis, from the Arendal area, southern Norway. The cells were slightly larger than Bonamia cells, always few, single, never associated with pathology or cellular response, and were not interpreted as Bonamia sp. However, in 2008, the EU reference laboratory received samples from the Norwegian Veterinary Institute and reported one Bonamia sp. in a haemocyte from one oyster. By real-time PCR, positive results were obtained from two oysters in one triplicate sample. On the background of this diagnose, both the National Veterinary Institute and Institute of Marine Research initiated an increased and targeted surveillance for Bonamia spp. in this oyster population. We have followed the population every year since 2009, and Bonamia has never been detected. More than 3000 oysters have been examined by histology and/or PCR, all with negative results. We have therefore considered the population as free from Bonamia spp. (Mortensen et al. 2016; 2020)
Studies on Marteilia pararefringens sp. nov. in blue mussels, Mytilus edulis
The blue mussel, Mytilus spp, populations are changing, and there are numerous reports on an un-explained “disappearance” or mortalities from many Nordic areas. It is not known to which extent diseases play a role in these changes. The wild mussels beds are not monitored on a regular basis, and there is a limited control of mussel farms, using traditional, extensive production methods based on the collection of wild seed.
The parasite Marteilia sp. was detected in mussels, Mytilus edulis, collected at Bømlo, western Norway in 2016. There is however a previous report of Marteilia in a paper describing histology of mussels in Rogaland, used as bioindicators of pollution (Aarab et al. 2011). Our finding at Bømlo was followed up with a survey in 2017 – 2018 and linked to several research projects. The results show that Marteilia sp. infecting mussels in Norway, Sweden and England are different from Marteilia refringens infecting flat oysters. The name Marteilia pararefringens has been proposed, and there is strong evidence that Marteilia refringens and Marteilia pararefringens sp. nov. are distinct parasites of bivalves and have different European distributions (Kerr et al. 2018).
Marteilia spp. do not transmit directly between bivalves. The life cycles include an alternate/intermediate host, which is suspected to be marine copepods (Audemard et al. 2002, Carrasco et al. 2007, Boyer et al. 2013, Arzul et al. 2014). The detection of M. pararefringens with high prevalence in the semi-closed, heliothermic oyster lagoon at Bømlo enabled us to study the infection cycle in the mussels, and the potential presence in associated fauna, including co-habitating flat oysters, O. edulis. The results are presently being prepared for submission (Bøgwald et al. in prep).
The surveillance programme for bonamiosis and marteiliosis in European flat oysters, Ostrea edulis, and blue mussels, Mytilus sp. in 2019
The surveillance programme for bonamiosis and marteiliosis in European flat oysters, Ostrea edulis, and blue mussels, Mytilus sp. is carried out by the Institute of Marine Research according to a contract with the Norwegian Food Safety Authority. The over-all aim is to gain knowledge on the health situation of farmed Norwegian oysters and mussels. There is however a connection between farmed and wild populations, due to the extensive nature of the bivalve industry. The work therefore requires a tight link between the Surveillance programme and the on-going research projects, as well as the building of a time-series of data. After the detection of Marteilia sp. in 2016, we increased the effort in order to study this case – including distribution and parasite life cycle. In 2019, we included three sites at Førlandsfjorden and Karmsundet; a re-visit to the sampling sites in the study of Aarab et al. (2011).
This report summarizes the results from the disease surveillance in 2019, gives a brief overview of the results from the studies on Marteilia pararefringes in mussels and presents some prospects for the work in 2020 and 2021.
2 - Material and methods
The surveillance was performed according to EU directive 2006/88 and Decision 2015/1554 . The sampling strategy, including wild beds and bivalve farms in operation, was revised in January 2015, and used as a background for the targeted surveillance also in 2019.
Sampling periods were defined according to the periods when the prevalence of Bonamia ostreae and Marteilia sp. (sporulating stage) are highest in the northernmost areas where they have been detected ( Engelsma et al . 2010; A. Alfjorden pers. comm ). The selected sampling sites are shown in Figure 1 and listed in Table 1.
Usually, the surveillance includes an on-site inspection, as the state of the population (density, reproduction, signs of mortality) are considered important meta-data. At Aga, Innerøy, Langestrand, Espevik, Hvaler, Haugevågen and Førlandsfjorden oysters and mussels were collected by skin-diving or wading in April and October and transported live to the Institute of Marine Research (IMR) in Bergen. At Lysefjorden, Åfjord and Rissa, the mussels were collected by the shellfish farmers and sent to IMR Bergen by over-night mail ( Table 1 ).
All oysters and mussels were processed at the IMR laboratory in Bergen, according to standard methodology, and under ISO 17025 QA. Briefly; Histology was performed using dorso-ventral cross sections, fixed in Davidson’s fixative, embedded in paraffin, sectioned at 3 µm, stained with Hematoxylin Eosin Saffron (HES), mounted with a cover slip and observed at 100 to 1000 x magnification. Samples for PCR were fixed in ethanol. DNA was extracted from ethanol fixed digestive gland tissue from mussels from Espevik. Marteilia refringens detection and typing was done with by Polymerase Chain Reaction as described by Le Roux et al. (2001). Samples where microcell-like structures were observed by microscopy were forwarded to real-time PCR as described by Corbeil et al . 2006 .
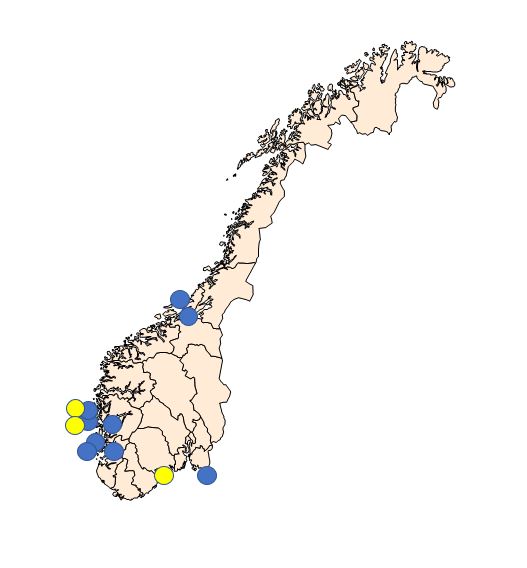
| Sampling site | Oysters | Mussels | |||
| Spring | Autumn | Spring | Autumn | ||
| Ytre Hvaler, Østfold | 32 | ||||
| Langestrand, Agder | 62 | An additional 90 samples are produced to comply with the requirement for 150 specimens | |||
| Lysefjorden, Rogaland | 30 | ||||
| Haugevågen, Rogaland | 15 | ||||
| Tysværvågen, Førlandsfjorden, Rogaland | 32 (inlet) , 32 (inner part) | ||||
| Aga Bømlo, Vestland | 30 | 27 | |||
| Innerøyen, Vestland | 30 | 30 | 30 | ||
| Espevik, Tysnes, Vestland. | 32 32 | Sampled by IMR Sampled by NFSA | |||
| Åfjord, Trøndelag | 31 | ||||
| Rissa, Trøndelag | 32 | ||||
3 - Results
Bonamia spp. was not observed in any sample in 2019. Marteilia sp. was not observed in oysters but were present in mussels from Bømlo and Tysnes. Results from the sites listed in Table 1 are briefly described below.
Examination of flat oysters
Langestrand, Agder
The site was inspected in May 2019. Dense oyster beds were observed down to approximately four-meter depth, with several cohorts present. There was no sign of abnormal mortality. Few adult Pacific oysters ( Crassostrea gigas ) were observed between the flat oysters. During sampling, Pacific oyster spat were observed on and in-between flat oyster shells and on pebbles in the inter-tidal zone.
Slides from 62 oysters have been read. The remaining will be read as a part of the process of applying for disease free status (see Mortensen et al . 2020 , Table 1). During examination, gross morphology of shells and soft parts appeared normal. In 19 slides a few structures resembling microcells were observed. Samples from these specimens were forwarded to PCR analysis. Intracellular (Rickettsial-like (RLO)) bacterial colonies were observed in digestive tissues in two specimens. Some neoplastic haemocytes were observed in two specimens.
Aga, Bømlo, Vestland
There is presently no production of flat oysters at the site, and the wild population fluctuates due to the environmental conditions. There is at present a relatively dense oyster population on abandoned suspended ropes and cultivation trays in the mid part of the lagoon. Condition index of the oysters was variable. Haemic neoplasia was observed in one oyster, and Intracellular Rickettsia-like colonies (RLO’s) were observed in the digestive epithelia of one oyster. Marteilia sp. was not observed.
Innerøyen, Vestland
Innerøyen is presently the only site in Norway with a traditional production of flat oyster spat.
Oysters were collected from suspended ropes in the mid part of the lagoon. The condition index of the oysters was low but variable. Intracellular Rickettsia-like bacterial colonies (RLO’s) were observed in the digestive epithelia of seven oysters (23%).
Examination of Mussels
Ytre Hvaler, Østfold
Mussels were collected from a low-density population on hard, shallow water bottom at Papperhavn, Ytre Hvaler. Mussels were in poor, but variable condition. Microcells were observed in connective tissues, in some cases associated with inflammations and green spots / pustules, numerous brown cells, focal necrosis and vacuolization of digestive epithelia, indicating release of lysozomes. These green spots were observed in mantle tissues of most mussels, and the infestations were considered infections with Coccomyxa parasitica (see Mortensen et al . 2005) . Most mussels were also moderately infected with trematode cysts.
Lysefjorden, Rogaland
Mussels were received from a commercial mussel farm in Lysefjorden, Rogaland. The farmer had reported high mortality of small/young (approx. 4 grams live weight) mussels. By microscopy, the mussels appeared low in meat content / energy reserves. Pathological alterations were observed in digestive tissues, with a high density of vacuoles, presumably lysosomes liberated from digestive epithelia into the lumina of diverticulae, which also contained an amorphic organic substance of un-known origin. There were increased densities of haemocytes, in some samples forming focal inflammations. Stomach epithelia generally appeared normal, and no pathogens were observed.
Haugevågen, Rogaland
Haugevågen is a sheltered lagoon on the north-western side of the island of Karmøy, in Rogaland. The lagoon and inlet have a dense population of flat oysters, and patches of mussels in some areas along the shoreline. The site was visited in October, together with a fisherman who harvests oysters in the area. He reported an acute mortality of mussels in the tidal current in the inner part of the inlet. Live mussels were collected by hand and transported back to IMR the same day. Mussels had variable condition. Eight (50%) had focal inflammations and/or infiltration of haemocytes in connective tissues and sinuses. One mussel had haemic neoplasia, five had low infestations with trematodes.
Marteilia infections in mussels were reported from two sites by Aarab et al . (2011) ; Tysværvågen in Førlandsfjorden and Høgevardane in Karmsundet. These two sites were re-visited in October 2019:
Tysværvågen, Førlandsfjorden, Rogaland
Tysværvågen is a large lagoon connected to Førlandsfjorden by a tidal current. Aarab et al . (2011) collected their mussels from rocks in the tidal current. We collected mussels from the same spot. There was a low-density population in the tidal current, dominated by large / old mussels. Areas in the inner part of the lagoon are dominated by influx of freshwater from small brooks/rivers. There were large, high density clusters of mussels on ropes and floating piers in the inner part. Mussels were collected also there, so the sampling included two sites from Tysværvågen.
Mussels from the tidal current were in good condition, with full energy reserves and variable but mainly full gonads. No pathogens or abnormal conditions were observed.
Mussels from the inner part of the lagoon were also in excellent condition, with a high meat content and full energy reserves. No pathogens or abnormal conditions were observed.
Høgevardane in Karmsundet
Høgevardane in Karmsundet has bays with sandy – clay bottom and rocky shoreline. The site is located near the effluent from an industrial aluminum melter. No live mussels could be found at the site.
Aga Bømlo, Vestland
The abandoned oyster lagoon at Aga, the short tidal current connecting the lagoon to the outside Håpollen and the inner part of Håpollen have been studied since 2017. The aim of the (still on-going) study is to unveil the infection and life cycle of Marteilia pararefringens in the local population of blue mussels. The site was visited in May and June 2019. Mussels collected in the inner part of Håpollen, close to the tidal current showed the same infection pattern as described in 2018, with 50 – 60 % Marteilia prevalence. Marteilia was present as secondary and tertiary (even in May!) stages. Most mussels were low in storage material and heavily infected with trematodes. Oysters from Aga were not infected by Marteilia (see above).
Espevik, Tysnes, Vestland
The oyster lagoon at Espevik is the most studied bivalve production site in Norway – a traditional “poll” (oyster lagoon) that was operated from the 1880’s until the 1990’s. During the last period of oyster production, it was owned by the same company who produced oysters at Aga. The lagoon has been abandoned for almost two decades, and today, there is a small population of flat oysters in the lagoon and some mussels in the inlet channel and outside. We had two Marteilia -PCR-positive samples from mussels collected in 2018 and re-visited the site in October 2019. Mussels were collected in the inlet. Mussels were in a poor condition and severely affected by marteiliosis. Marteilia prevalence was 88% and found in a sporulating stage, with necrosis of digestive mussel tissues and heavy haemocyte infiltrations. PCR and sequencing verified the presence of M. refringens Type M / M. pararefringens . The Norwegian Food Safety Authority was notified. Inspectors from the Norwegian Food Safety Authority collected a new sample from a pier approximately 200 meters from the inlet in January 2020. One mussel was infected. Intracellular bacteria (RLO-like) was observed in one mussel. One mussel had a severe haemic neoplasia and was in a moribund stage.
Innerøyen, Vestland
Innerøyen is presently the only site in Norway with a traditional production of flat oyster spat.
During the last years, the fauna in the lagoon has been dominated by mussels, which the oyster farmers are actively removing. The lagoon has conditions that are comparable to Aga and Espevik and may therefore potentially inhabit the alternate/intermediate host for Marteilia pararefringens . Mussels and oysters (see above) were collected in October. Mussels appeared low in energy reserves and with empty gonads. Two mussels had haemic neoplasia. Marteilia sp. was not detected.
Åfjord, Trøndelag
Mussels from Åfjord come from a commercial mussel farm. Mussels appeared in variable to good condition. No abnormal finding was recorded.
Rissa, Trøndelag
Mussels from Rissastrømmen come from a wide tidal current used as intermediate grow-out and harvest of farmed mussels. Mussels appeared in good but variable condition. No abnormal finding was recorded.
4 - Discussion and conclusions
The health status of flat oysters
Healthy flat oysters – which in the present situation particularly means free from Bonamia spp. - is a valuable resource, both for European oyster growers, as well as programmes aiming at re-stocking the disappearing, historical wild oyster beds. It is an important task to monitor Norwegian stocks and disseminate the information on their health status in order to obtain a consensus on how to protect and care for this resource. The institute of Marine Research has started a monitoring of Norwegian flat oyster populations and aim at using the data gained in a Nordic and European context. The monitoring of stocks is linked to the national health surveillance, and through the contact with European scientists to both genetic studies ( https://interreg-oks.eu/projektbank/projekt/margeniikapacitetsopbygningforogetbaredygtigvakstvedakvatiskemiljoerikattegatskagerrak.5.19de82b216b0032caaeda5ad.html ) and re-stocking programmes ( https://noraeurope.eu/ ). Flat oysters from Hafrsfjord in Rogaland has already been seeded off the coast of The Netherlands.
The wild flat oyster populations examined in 2019 appear healthy, with a normal reproductive cycle pattern. Haemic neoplasia and the presence of intracellular Rickettsia-like colonies were occasionally observed, but at low prevalence and intensity. This is a common observation, and not considered a problem, although the neoplasia may develop into disease problems and potentially induce winter mortalities of affected flat oysters as their nutrient uptake and immune defense are knocked out ( Mortensen et al . 2013 ).
The oysters from Aga have a relatively poor condition index, probably due to food limitation. In 2019, Marteilia spp. has not been detected in these oysters, neither by histological examination nor PCR, although the oysters were collected in the lagoon, together with the Marteilia -infected mussels. Previous samples from oysters and mussels collected at Rogøysund – a farm receiving oyster spat from Aga – have been negative, indicating that M. pararefringens has not been moved to oyster farms with oyster spat from the poll. The results strengthen the common finding that M. pararefringens does not infect flat oysters.
At Langestrand, several cohorts have been present throughout the study period. All samples since 2008 have been Bonamia negative ( Mortensen et al . 2016, Mortensen et al . 2020 ). The situation has thus been stable since 2006. A 13 year-long sub-clinical Bonamia infection seems unlikely, taking into account that this oyster bed experiences extremely variable conditions through the seasons.
Examination of mussels and the distribution of Marteilia pararefringens sp. nov.
M. pararefringens was detected in Aga in 2016, and in a lagoon in Espevik, Tysnes (during the present study) in 2019. Both these sites are small, heliothermic, traditional oyster lagoons, with similar conditions. We have previously shown that M. pararefringens DNA is present in zooplankton in the lagoon at Aga, and probably, the Espevik lagoon also represents an environment where the intermediate host is present, and the Marteilia life cycle may be completed. However, Marteilia sp. has so far not been detected in the lagoon at Innerøyen. This indicates that M. pararefringens may be present in some, but not all, the traditional oyster lagoons along the coast. Mussels from the site at Tysværvågen in Rogaland, where Marteilia was detected by Aarab et al . (2011) were Marteilia negative during our re-visit in 2019. The density of mussels in the inlet was low, and it is possible that Marteilia may have caused a mortality, leaving mainly un-infected mussels, or surviving mussels with a low infection intensity. The sample size may thus have been too low to detect a low intensity, low prevalence infection. In July 2020 we detected high prevalence of M. pararefringens in two wild oyster beds in Flekkefjord in Sunnfjord, with a potential drift of Marteilia to a near-by mussel farm. This finding represents a new situation, where the parasite occurs outside a closed lagoon environment. In the continuation of this study, we will look more closely at the traditional oyster lagoons, mussels in closed, sheltered areas and try to understand the distribution and possible movements of M. pararefringens in the mussel populations. In order to optimize the sampling regime in the surveillance programme, we will collaborate with all active mussel producers in order to map the farms, spat collection areas and translocations.
The Norwegian mussel production is concentrated in Trøndelag and Nordland, where two dispatch centers receive mussels from several producers in a large geographic area. Although two selected sites are included in the surveillance programme, we do not have a proper overview of the production in this area. The dispatch centers will be followed up and their sources of mussels mapped. The situation illustrates the need for combining the surveillance with the health control of shellfish farms.
The life history of M. pararefringens from the time of sporulation (autumn) until re-infection of mussels (summer) is still un-known. PCR-analysis of fauna samples gave positive results in samples from plankton sampled during summer. The studies will be continued in 2021, focusing on plankton, other invertebrate fauna, water and sediment in the period May – July.
It is important to be sure that oysters are not susceptible to M. pararefringens or may act as vectors. We will thus continue to combine surveillance and research activity in order to obtain as much data as possible, also from oysters at the infected site, and from more sites that have been in contact with the former network of oyster producers.
We have not uncovered the causes of the mortalities of mussels from the farm in Lysefjorden and the wild population in Haugevågen. Pathological changes in the mussels from Lysefjorden could have been a result of external stressors causing a response in digestive tissues and release of lysosomes.
Conclusions and recommendations
Revision of the programme. Selection of sites and increased sample size
The surveillance will be continued according to the plan discussed with the Norwegian Food Safety Authority, and with the aim of obtaining a time series of data on the health status of flat oysters and mussels along the Norwegian coast. We will combine surveillance, research activities and monitoring of wild populatios in order to optimize the collection of data.
The surveillance programme will be focused on mussel and oyster farms and/or populations that are commercially harvested for on-growth, transport to the markets or used in re-stocking programmes.
The detection of Marteilia pararefringens in mussels in new areas indicate that this parasite is more widely distributed than previously known. We consider the sample size of 30 specimens (or 20 as applied in the Veterinary health control of commercial farms) as too low. This sample size should only be used if the health status is known, after a previous two-year surveillance based on 150 specimens every 6 months, based on the principles laid down in EU Decision 2015/1554 . To improve the screening and ensure a rapid detection, we propose – and will apply - a method based on 100 specimens per sampling, whereas 50 will be analyzed by PCR and 50 by histology.
Collaboration in order to obtain better data
The health surveillance will be linked to monitoring of stocks, oyster genetic studies and European oyster projects, strengthening the scientific basis for a strong and adequate management of the few remaining, healthy flat oyster populations in Europe.
Linking surveillance, health control and scientific studies
We will outline a new, risk-based model for health monitoring of mussels and oysters which combines surveillance and health control.
Knowledge on Marteilia pararefringens – progress of the work
Flat oysters do not appear susceptible to M. pararefringens. Future scientific studies will focus on understanding the infection and life cycle of M. pararefringens in mussels, as a background for a risk assessment and an epidemiological study of the spreading of this parasite in North European mussel populations.
Categorization of mussel and flat oyster production areas in Norway
The samples from Trøndelag collected in 2018 and 2019 represent the first study of the health status of mussels north of Bergen. Due to the lack of data on the health status of mussels from most of the Norwegian coast, the categorization should be discussed and potentially revised.
Absence of Bonamia spp. in Norway
Bonamia spp. is still absent in the flat oyster population at Langestrand, Agder. This population will be excluded from the Surveillance program from 2020 and onwards. Sampling will however be done if required by the Norwegian Food Safety Authority. The previous reports (from 2016 and 2020) as well as the results presented in this report may be used as background data for of the process of applying for declared freedom for bonamiosis.
Acknowledgements
Thanks to Ingrid U. Fiksdal, Dawit B. Ghebretnsae and Håkon Berg-Rolness for technical assistance, to Øivind Strand for help with sampling in the field, to David Bass for the collaboration on the M. pararefringens life cycle and to Renee Beckman for information about the sampling by Aarab and co-workers.
5 - References
Abollo E, Ramilo A, Casas SM, Comesaña P, Cao A, Carballal MJ, Villalba A (2008). First detection of the protozoan parasite Bonamia exitiosa (Haplosporidia) infecting flat oyster Ostrea edulis grown in European waters. Aquaculture 274: 201-207.
Arab, N., Godal, B.F., Bechmann, R.K. (2011). Seasonal variation of histopathological and histochemical markers of PAH exposure in blue mussel ( Mytilus edulis L.). Marine Environmental Research 71: 213-217.
Arzul I, Chollet B, Boyer S, Bonnet D, Gaillard J, Baldi Y, Robert M, Joly J-P, Garcia C, Bouchoucha M (2014). Contribution to the understanding of the cycle of the protozoan parasite Marteilia refringens . Parasitology 141(2): 227-240.
Audemard C, Le Roux F, Barnaud A, Collins CM, Sautour B, Sauriau PG, de Montaudouin X, Combes C, Berthe FJC (2002). Needle in a haystack: Involvement of the copepod Paracartia grani in the life-cycle of the oyster pathogen Marteilia refringens . Parasitology 124: 315-323.
Boyer S, Chollet B, Bonnet D, Arzul I (2013). New evidence for the involvement of Paracartia grani (Copepoda, Calanoida) in the life cycle of Marteilia refringens (Paramyxea). International Journal for Parasitology 43(14): 1089-1099.
Bøgwald M, Skår CK, Karlsbakk E, Alfjorden A, Feist SW, Bass D, Mortensen S (202_). The infection cycle of Marteilia pararefringens in blue mussels, Mytilus edulis in a Norwegian heliothermic marine lagoon. Manuscript in prep.
Carrasco N, López-Flores I, Alcaraz M, Furones MD, Berthe F, Arzul I (2007). Dynamics of the parasite Marteilia refringens (Paramyxea) in Mytilus galloprovincialis and zooplankton populations in Alfacs Bay (Catalonia, Spain). Parasitology 134-11: 1541-1550.
Corbeil S, Arzul I, Robert M, Berthe FCJ, Besnard-Cochennec N, Crane MSJ (2006). Molecular characterization of an Australian isolate of Bonamia exitiosa . Diseases of Aquatic Organisms 71:82-85.
Elston RA, Farley CA, Kent ML (1986). Occurrence and significance of bonamiasis in European flat oysters Ostrea edulis in North America. Diseases of Aquatic Organisms 2:49-51.
Engelsma MY, Kerhoff S, Roozenburg I, Haenen OLM, van Gool A, Sistermans W, Wijnhoven S, Hummel H (2010). Epidemiology of Bonamia ostreae infecting European flat oysters Ostrea edulis from Lake Grevelingen, The Netherlands. Marine Ecology Progress Series 409: 131 – 142.
EU. Council directive 2006/88/EC of 24 October 2006 on animal health requirements for aquaculture animals and products thereof, and on the prevention and control of certain diseases in aquatic animals. Official Journal of the European Union L 328/14.
EU. Decision 2015/1554 of 11 September 2015, laying down rules for the application of Directive 2006/88/EC as regards requirements for surveillance and diagnostic methods. Official Journal of the European Union L 247/1.
Kerr R, Ward GM, Stentiford GD, Alfjorden A, Mortensen S, Bignell JP, Feist SW, Villalba A, Carballal MJ, Cao A, Arzul I, Ryder D, Bass D (2018). Marteilia refringens and Marteilia pararefringens sp. nov. are distinct parasites of bivalves and have different European distributions. Parasitology 1–10. https://doi.org/10.1017/ S003118201800063X
Le Roux F, Lorenzo G, Peyret P, Audemard C, Figueras A, Vivares C, Gouy M, Berthe F (2001). Molecular evidence for the existence of two species of Marteilia in Europe. Journal of Eukaryote Microbiology 48: 449-454.
Mortensen S, Harkestad LS, Stene R-O, Renault T (2005). Picoeucaryot alga infecting blue mussel Mytilus edulis in southern Norway. Diseases of Aquatic Organisms, 63:25-32.
Mortensen S, Skår CK, Harkestad LS (2013). Hemisk neoplasi hos norsk flatøsters, Ostrea edulis . Norsk Veterinærtidsskrift 125 (7): 438 -442.
Mortensen S, Sælemyr L, Skår CK, Bodvin T, Jelmert A (2016). Health surveillance of the flat oyster populations in Aust-Agder County, southern Norway in the period 2009 – 2015. Rapport fra havforskningen nr 11, 2016, 11s.
Mortensen S, Skår C, Sælemyr L (2020). Summarizing the screening for Bonamia ostreae in Norwegian populations of flat oysters, Ostrea edulis . Rapport fra Havforskningen no 24, 2020. ISSN:1893-4536, https://www.hi.no/hi/nettrapporter/rapport-fra-havforskningen-en-2020-24