This report summarizes relevant peer-reviewed and published knowledge on effects (physical, biogeochemical, biological) of bottom trawling and Scottish seining on benthic habitats and fauna. Emphasis is placed on the effects of groundfish and shrimp trawling relevant for Norwegian fisheries. The report describes the best available knowledge of the occurrence of vulnerable habitats in Norwegian waters. Maps are presented showing the distribution of fisheries utilising bottom trawl and Scottish seine. Bottom trawling is the most widespread human activity affecting seabed habitats. Otter boards (trawl doors), clump weights (for multiple trawl deployment) and groundgear penetrate the substratum, resuspend sediment and generate plough marks, usually <30 cm deep, while the sweeps have the largest footprint in terms of area. The degree and longevity of physical disturbance depend on the type of trawl (e.g. type otter board and groundgear), vessel speed, type of substrate, currents, wave action and biological activity. Impact is greatest on soft sediments consisting of silt and clay. Remineralisation of organic carbon (of terrestrial or marine origin) stored in sediments, i.e. the transformation into inorganic carbon (e.g. CO2 ), can be impacted by bottom trawling. These are complex processes interacting with sediment type, organic carbon content, biology and hydrodynamics (currents and wave action). Quantification of remineralisation resulting from bottom trawling will require site specific sampling and detailed examination. Biological effects of bottom trawling on soft sediments may be difficult to quantify due to temporal and spatial heterogeneity in bottom fauna (i.e. natural variations). Scientific long-term studies designed to reveal such effects unequivocally need rigorous before-after control-impact (BACI) experimental designs. To date, there are few examples of studies designed to examine the impact of Norwegian bottom trawl fisheries on benthic habitats and fauna, or on the ecosystem associated with the seafloor. Such effects will depend on gear type, trawling intensity, type of substrate, species assemblages and hydrodynamics. However, impacts of bottom trawling are well established for biogenic habitats dominated by large-bodied, sessile, slow growing and long-lived organisms (e.g. solitary corals, cold water coral reefs, sponge gardens, sea fans, sea whips). Studies conducted in the Barents Sea have shown that bottom trawling by-catches, moves and damages benthic fauna in such habitats. Studies in other seas and habitats have demonstrated reduction in abundance and biomass of examined species. The degree of impact varies between studies (i.e. effects are site-specific). Rates of recovery for habitats impacted by bottom trawling vary according to trawling intensity and duration of impact, species assemblage and hydrodynamics. Soft bottom fauna will show much faster recovery than coral reefs. Moderately impacted areas may recover within one year, whereas for areas where bottom trawling has occurred over longer time periods recovery times may exceed 10 years. There are significant knowledge gaps concerning the impact of bottom trawling on benthic habitats, species assemblages and ecosystems associated with the ocean floor. Large-scale and site-specific replicated management experiments are urgently needed to generate relevant knowledge on the effects of bottom trawling and the significance/value of restored bottom fauna communities for the ecosystem and its functioning.
Effekter av bunntråling
— Sammenstilling av kunnskap om bunnpåvirkning fra trål og snurrevad relevant for norske farvann
Rapportserie:
Rapport fra havforskningen 2023-1
ISSN: 1893-4536
Publisert: 31.01.2023
Oppdatert: 31.03.2023
Prosjektnr: 15859
Oppdragsgiver(e): Nærings- og Fiskeridepartementet
Referanse: Tildelingsbrev 2022 - Havforskningsinstituttet
Forskningsgruppe(r):
Fangst
Tema:
Hav, kyst og fjord ,
Marint vern
Program:
Barentshavet og Polhavet
Approved by:
Research Director(s):
Geir Huse
Program leader(s):
Maria Fossheim
English summary
Sammendrag
Rapporten gir en sammenstilling av publisert kunnskap om hvordan bunnhabitater og bunndyr påvirkes (fysiske, kjemiske og biologiske effekter) av fiske med bunntrål og snurrevad. Det legges hovedvekt på påvirkninger fra fisketrål og reketrål som er relevant for norske forhold. Rapporten beskriver utbredelsen av sårbare habitater i norske havområder, og det presenteres kart som viser hvor det fiskes med fisketrål, reketrål og snurrevad. Bunntråling er den menneskelige aktiviteten som i geografisk utstrekning har størst påvirkning på havbunnen. Fiske med bunntrål fører til oppvirvling av sedimenter, og tråldører og senterklump (for dobbel- og trippeltrål) lager spor på bunnen som er inntil 30 cm dype, mens det er sveipene som arealmessig gir størst bunnpåvirkning. Graden og varigheten av fysisk påvirkning avhenger av type bunntrål (f.eks. type tråldører og bunngir), fartøyets fart, type sedimenter, strømforhold, bølgeaktivitet og biologisk aktivitet. Effektene er størst på bløt mudderbunn bestående av leire og silt. Remineralisering av organisk karbon (med marint eller terrestrisk opphav) lagret i sedimenter, dvs. omdannelse til uorganisk karbon (f.eks. CO2), kan påvirkes av bunntråling. Dette er komplekse prosesser som bestemmes av interaksjoner mellom en rekke faktorer (bl.a. type sedimenter, mengde organisk karbon, biologisk aktivitet, strøm og bølgeaktivtet). Kvantifisering av remineralisering som følge av bunntråling i norske farvann vil kreve stedsspesifikke og detaljerte undersøkelser. Biologiske effekter av bunntråling på bløte sedimenter kan være vanskelig å kvantifisere fordi mange bunndyrsamfunn er heterogene og viser naturlige variasjoner i tid og rom. Vitenskapelig dokumentasjon krever at det gjennomføres før/etter studier der påvirkede områder sammenlignes med upåvirkede kontrollområder eller områder der påvirkningen opphører. Det er utført få studier av hvordan norske trålfiskerier påvirker bunnhabitater og bunnfauna, og slike effekter vil være påvirket av forhold som redskapstype, intensiteten i fiskeriet, type bunnhabitat, type substrat, artssammensetning og naturlige forstyrrelser (havstrømmer og bølger) i hydrodynamisk aktive områder. Det er godt dokumentert at bunntråling har stor påvirkning på habitater dominert av store, fastsittende, sentvoksende og langtidslevende organismer som koraller, svamper og sjøfjær. Undersøkelser utført i Barentshavet har vist at tråling fanger, forflytter og skader bunnfauna i slike habitattyper. Studier i andre havområder og på andre typer bunnhabitater har vist nedgang i antall og biomasse av noen av de undersøkte artene, og at graden av påvirkning varierer mellom studiene (dvs. effektene er stedsspesifikke). Restitusjonstiden for habitater som har vært påvirket av trålaktivitet vil variere mye avhengig av fiskeintensitet og varighet, type bunndyrsamfunn og naturlig påvirkning fra strøm og bølger. Et bløtbunnsamfunn vil restitueres langt raskere enn et korallrev, og studier har vist at moderat påvirkete områder kan restituere i løpet av ett år, mens for områder hvor det har pågått tråling i mange år vil restitusjonstiden være lengre enn 10 år. Det er store kunnskapshull om effektene av bunntråling på ulike bunnhabitater og bunndyrsamfunn, og på økosystemet knyttet til havbunnen. Storskala og stedsspesifikke forvaltningseksperimenter vil være nødvendige for å frembringe relevant kunnskap om effekter av bunntrål og betydningen av restituerte bunnsamfunn for økosystemet og dets funksjoner.
Oppdatert 31.03.2023: Rettet feil i figurene 2.1 og 2.2 og den medfølgende teksten.
1 - Introduksjon
1.1 - Oppdraget/bestillingen
I Nærings- og Fiskeridepartementet (NFD) sitt tildelingsbrev til Havforskningsinstituttet (HI) for 2022 står det under prioriterte tiltak at forvaltningen har «behov for vitenskapelig kunnskap om bunnpåvirkning fra trål, relevant for norske forhold, både når det gjelder miljøpåvirkning og hvorvidt restitusjon er mulig og hensiktsmessig.» Oppdraget er konkretisert som en bestilling i tildelingsbrevet der det står at HI skal «Lage en sammenstilling om publisert kunnskap om virkningene av bunnfiske, som er relevant for fiskerier i norske farvann.»
Bakgrunnen for bestillingen er at økt næringslivsaktivitet medfører at forvaltningen har behov for mer kunnskap om det marine miljøet for å oppnå best mulig sameksistens mellom ulike næringer. Forvaltningen har også behov for støtte til arbeidet under Konvensjonen om biologisk mangfold (CBD) og oppfølging av Stortingsmelding 29 (2020-2021) om vern av det marine miljø ("Heilskapeleg plan for bevaring av viktige områder for marin natur"). Kunnskap om hvordan menneskelig aktivitet påvirker miljøet vil stå sentralt i vurderingen av hvilke fiskeriforvaltingstiltak som kan sikre bevaring av viktige og sårbare marine områder.
Bunntråling er den menneskelige aktiviteten som i geografisk utstrekning har størst påvirkning på havbunnen (Hiddink et al., 2017; Sciberras et al., 2018). I dype områder der havbunnen i liten grad påvirkes av strøm og bølger (mindre hydrodynamisk aktive områder) er bunntråling antatt å være den viktigste mennskelige påvirkningen på sedimenteringsprosesser (Martín et al., 2014). Internasjonalt er det utført en rekke vitenskapelig studier av hvordan bunntråling påvirker sedimenter og habitater, mens det er utført få undersøkelser i norske farvann. De fleste studiene har fokusert på kartlegging av det fysiske fotavtrykket (areal og penetrasjonsdyp) av ulike bunnredskaper (Eigaard et al., 2016; 2017) og på endringer i biodiversitet (artssammensetning, tetthet, biomasse) som følge av påført skade og fjerning av bentisk fauna (Hiddink et al., 2017; Sciberras et al., 2018; Mazor et al., 2020). I en nylig publisert vitenskapelig artikkel (Sala et al., 2021) som har fått stor oppmerksomhet, hevdes det at bunntråling også fører til remineralisering av organisk karbon lagret i bunnsedimenter og dermed frigivelse av CO2.
Rapporten støtter seg i hovedsak på internasjonale fagfellevurderte arbeider når den omhandler effektene av fiske med fisketrål, reketrål og snurrevad som er de redskapene som i størst grad påvirker havbunnen i norske farvann. Det gis en oversikt over de havområdene hvor den norske fiskeflåten opererer. Kart som viser områder med høyt fiskepress sammenstilles med kart som viser bunntype (sedimentkart) og sårbare områder. Rapporten beskriver hvordan bunnhabitater påvirkes av fysiske, kjemiske og biologiske effekter forårsaket av bunntråling. Videre gis det en vurdering av restitusjonstid for ulike bunnsamfunn, dvs. hvor lang tid det tar før bunndyrsammensetningen er gjenoppbygd og lik et tilsvarende upåvirket habitat.
I dag finnes det gode data på hvor fiskeflåten (fartøy > 15 m) opererer (loggbok-data og VMS-data), mens kunnskap om effektene av bunnfiske (dødelighet og skadeomfang) og restitusjonstid for bunnfauna er mangelfull (Hiddink et al., 2017). Økt kunnskap på dette feltet er en forutsetning for bærekraftig forvaltning av det marine miljøet.
1.2 - Hvilke fiskeredskaper påvirker bunnen?
Fiskeredskaper deles inn i de to hovedgruppene aktive/mobile og passive/stasjonære. Det er de mobile redskapene med bunnkontakt som regnes å ha klart størst påvirkning på bunnsedimenter og bunnsamfunn (Eigaard et al., 2016). Denne redskapsgruppa består av bunntrål, snurrevad, bomtrål og ulike typer skjellskraper. Fotavtrykk og påvirkning fra andre mobile redskaper (f.eks. ringnot) og stasjonære redskaper (line, teine, garn) vil være ubetydelig i forhold.
I norske farvann er bunntrål og snurrevad svært utbredt, mens skraper er tilnærmet ikke-eksisterende og det er totalforbud mot fiske med bomtrål. Bomtrål og skraper har stor fysisk påvirkning (penetrasjonsdyp), og studier har vist en sammenheng mellom penetrasjonsdyp og graden av påvirkning (Hiddink et al., 2017; Sciberras et al., 2018). På bakgrunn av økende fokus på effektene av bunntråling (Eigaard et al., 2016; Hiddink et al., 2017; Mazor et al., 2020), er det lite sannsynlig at det vil åpnes for nye norske fiskerier basert på bomtrål og skraper. Denne rapporten sammenstiller derfor publisert kunnskap om påvirkningene av fiske med bunntrål (fisketrål og reketrål) og snurrevad.
2 - Bunntrålfiskerier i norske farvann
2.1 - Tekniske beskrivelser av bunntrål og snurrevad
Detaljerte beskrivelser og illustrasjoner av ulike typer bunntrål og snurrevad er gitt av He et al. (2021).
2.1.1 - Fisketrål
Bunntrål er et konisk utformet nett som slepes langs bunnen. Trålen holdes åpen i vertikalplanet ved hjelp av trålkuler (kuletelne) og et bunngir (grunntelne) og i horisontalplanet av to tråldører. Tråldørene er festet til selve trålen ved hjelp av sveiper. Bunntrål brukes i fiskerier etter en rekke bunnfiskarter (torsk, sei, hyse, uer, tobis, kolmule, øyepål), reke og sjøkreps.
I Barentshavet er det vanlig å bruke dobbeltrål i fiske etter torsk og hyse (Selstad 640 eller Vonin/Mørenot 738). I tillegg til de to tråldørene brukes det da ei tung vekt (senterklump) mellom de to trålene. Vanlig oppsett for en dobbeltrål er 160 m lange sveiper, 320 m dørspredning, 24 tommers rockhopper gir, 6,5 tonns senterklump og 12 m2 (ca. 3 tonn) dører.
I fisket etter sei i Nordsjøen bruker de fleste båtene dobbeltrål med samme rigging som torskefisket i Barentshavet. Noen båter bruker enkeltrål og pelagiske dører. Det benyttes da 80 m sveiper, ei vekt (ca. 1,2-1,4 tonn) 40 m bak dørene og en dørspredning på 125 m.
Tråler til tobisfisket leveres i flere størrelser avhengig av båtstørrelse og tauekraft. Det brukes kjetting som bunngir (vanligvis 160 m lange 19 mm kjettinglenker, ca. 1200 kg) og 100 m sveiper. Døravstanden er ca. 140 m og det er mest vanlig å bruke semipelagiske dører (4-5 tonn, Tornado fra Vonin, 11 m2 eller Type 22 fra Thyborøn, 13 m2).
I industritrålfisket er det vanlig å bruke en Expo trål der størrelsen er tilpasset båtstørrelsen. Enkelte båter bruker samme type trål rigget som dobbeltrål. Det brukes en lett 13 mm kjettinglenke (60 m, ca. 225 kg) som bunngir og 150 m sveiper. Industritrålerne bruker semipelagiske dører (Thyborøn Type VF15, 8,5 m2, 2 tonn) med en døravstand på rundt 160 m.
2.1.2 - Reketrål
I reketrålfisket i Barentshavet bruker noen båter fortsatt dobbeltrål, men de fleste har gått over til trippeltrål. Det brukes da to senterklumper (en på hver side av den midtre trålen), som hver veier 7-9 tonn. Det brukes 21-24 tommers rockhopper gir, 18 m2 dører (ca. 6 tonn), og en dørspredning på 160 m.
Flåten som fisker langs kysten og i Nordsjøen og Skagerrak består av fartøyer fra ca. 10 m til knapt 40 m. De fleste reketrålerne fisker med dobbelttrål, mens en del mindre fartøy fisker med enkelttrål. Ett fartøy har begynt å fiske med kvadruppeltrål (4 tråler samtidig). De minste fartøyene fisker kystnært.
Tråldører varierer i vekt fra 500 til 2000 kg. Senterklump på dobbelttrål veier ca. 70% mer enn vekten av tråldørene. Døravstanden er ca. 50 m for enkelttrål og 180 m for dobbelttrål og sveipene er fra 30 til 50 m. Kvadruppeltrålen er rigget med 4 tråldører og en midtklump. Ytterst brukes det 140" Thyborøn type 2 dører med en vekt på 2 000 kg, mens det innerst brukes to 100" dører type 11 med en vekt på 1800 kg. Midtklumpen er på 2,5 tonn. Sveiper/haneføtter er på 38 m som gir en døravstand på ca. 220 m.
Kuletelne med 8" plastkuler er mest brukt blant mindre og middelstore fartøy. Større fartøy bruker 11" kuler, og i Skagerrak brukes det også 12" kuler. Med større kuler går redskapen lettere på bløt bunn som dominerer i østre deler av Skagerrak. Noen mindre fartøy (spesielt på Sør-Vestlandet) bruker "sabb" (trosse) med kjetting som bunngir.
2.1.3 - Snurrevad
Snurrevad er, i likhet med trål, et konisk utformet nett som slepes langs bunnen. Som for trål, holdes snurrevaden åpen i vertikalplanet ved hjelp av en kuletelne og en grunntelne. Snurrevad har ikke tråldører og sveiper, men lange tau (opptil 2600 m) som settes slik at nettet (nota) holdes åpen i horisontalplanet. Snurrevad brukes i fiske etter torsk, hyse, sei, flyndre og i noen grad blåkveite.
Langs grunntelnen er det som regel festet et «skjørt» som er like langt som grunntelnen. Skjørtet danner en «nettvegg» for å hindre at selve snurrevaden går i bunn og skades. Skjørtet er laget av kvadratiske masker med en høyde på 0,5-1 m (større snurrevader har som regel høyere skjørt). Den nedre delen av skjørtet har som oftest en kjetting som sørger for bunnkontakt. Forhold mellom fløyt og vekt er slik at kjettingens vekt er større enn fløytenes oppdrift. Denne forskjellen varierer fra ca. 70 kg og opp til 600 kg, og justeres blant annet på grunn av strømforhold.
Snurrevadtaua er kombinasjonstau (dvs. en kjerne av stål med en ytterkappe av plast). De større båtene bruker tau med en diameter på 40-60 mm, mens dimensjonen er 28-36 mm hos de mindre båtene. Vekten på snurrevadtauene varierer mellom de ulike fiskeriene (artene) og etter båtstørrelse og taulengde, men det er en tendens til stadig tyngre tau i snurrevadfiske.
Snurrevad brukes i økende grad i fiske etter sei. I seifiske kreves det større hastighet og dermed tyngre gir enn i torske- og hysefiske. I fiske etter sei taues det gjerne med ca. 3 knops hastighet, mens i torskefiske kan hastigheten være så lav som 0,7 knop. Taulengden er også lengre i seifisket. Disse forholda krever mer motorkraft, og det er derfor hovedsakelig de større snurrevadbåtene som deltar i seifiske.
Snurrevadfiske foregår i stor grad i kystnære strøk hvor det i henhold til Høstingsforskriften er satt begrensninger på dimensjonene til redskapen. De fleste båtene har derfor snurrevader med maksimal tillatt størrelse (se pkt. 2.2.2 nedenfor). De mindre båtene har da gjerne snurrevader laget av tynnere og lettere materiale enn de større båtene, mens de aller minste båtene har noe mindre snurrevader.
2.2 - Geografisk avgrensning i fiske med bunntrål og snurrevad
2.2.1 - Fisketrål og reketrål
I henhold til Havressurslova (Lov om forvaltning av viltlevende marine ressurser) er det forbudt å fiske med trål innenfor territorialgrensa (12 nautiske mil (n.mil) fra grunnlinjene). I Høstingsforskriften (Forskrift for gjennomføring av høsting av viltlevende marine ressurser) er det gjort følgende unntak fra dette forbudet:
-
Fartøy med en bruttotonnasje inntil 1200 kan fiske med stormasket bunntrål i området mellom 6 og 12 n.mil fra grunnlinjene. Fangsten skal landes for bearbeidelse ved anlegg i Norge i fersk, frosset eller saltet tilstand og kan ikke være filetert.
-
Fartøy under 34 meter største lengde kan fiske vassild og kolmule med småmasket bunntrål i området mellom 6 og 12 n.mil fra grunnlinjene sør for 67° 10′ N.
-
Det er tillatt å fiske med reketrål (småmasket bunntrål) innenfor 12 n.mil fra grunnlinjene, men det er forbudt å bruke rockhopper gir. Innenfor fjordlinjene er det tillatt å fiske med reketrål for fartøyer under 19,81 m. Innenfor grunnlinjene det er forbudt å fiske med reketrål på grunnere vann enn 60 m fra svenskegrensa til Jærens rev, grunnere enn 100 m fra Jærens rev til og med Trøndelag fylke og grunnere enn 170 m nord for Trøndelag.
-
Det er tillatt å fiske sjøkreps med stormasket trål i området mellom 4 og 12 n.mil fra grunnlinjene og innenfor 4 n.mil fra grunnlinjene i Skagerrak (øst for Lindenes fyr).
I Forskrift om regulering av fiske for å beskytte sårbare marine økosystemer og Forskrift om beskyttelse av korallrev mot ødeleggelser som følge av fiskeriaktivitet er det listet opp en rekke områder der det er forbudt å fiske med bunnredskaper.
2.2.2 - Snurrevad
Det et generelt forbud mot å bruke rockhopper gir i snurrevad og det er forbudt å bruke bobbinslenke nord for 62° N.
I området innenfor 4 n.mil fra grunnlinjene er det forbudt å bruke snurrevad som har en kuletelne eller grunntelne lengre enn 123 m, en omkrets i åpningen større enn 156 m eller mer enn 2000 m taulengde.
Det er forbudt å bruke snurrevad innenfor fjordlinjene, men det er gjort enkelte unntak for fartøy under 11 m (jfr.§ 32 i Høstingsforskriften).
2.3 - Hvor fiskes det med bunntrål og snurrevad i norske farvann?
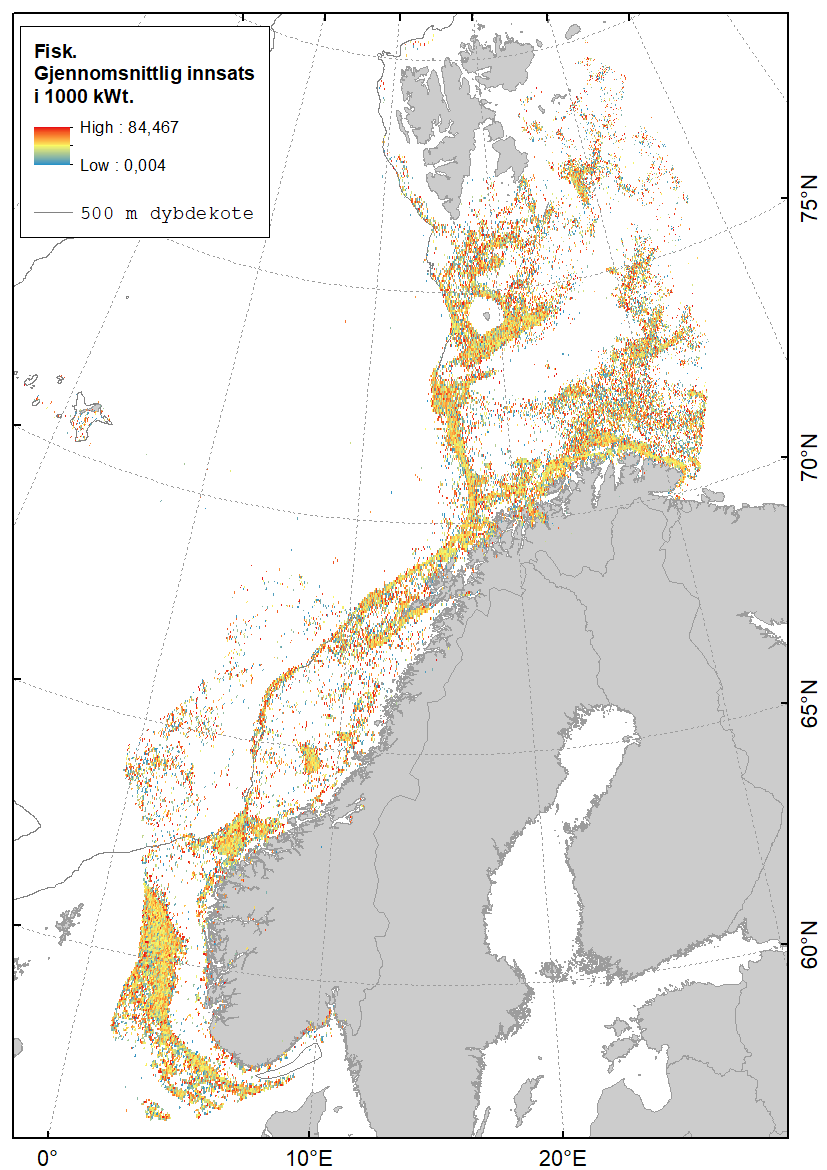
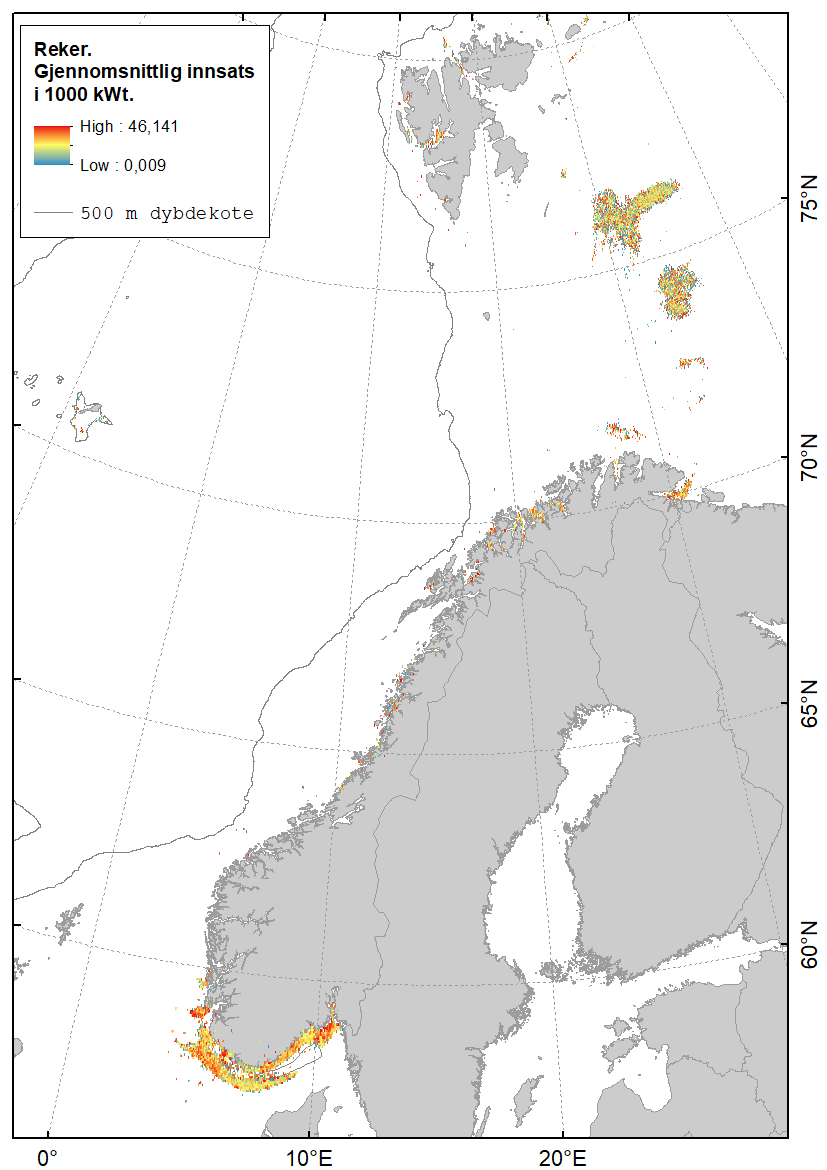
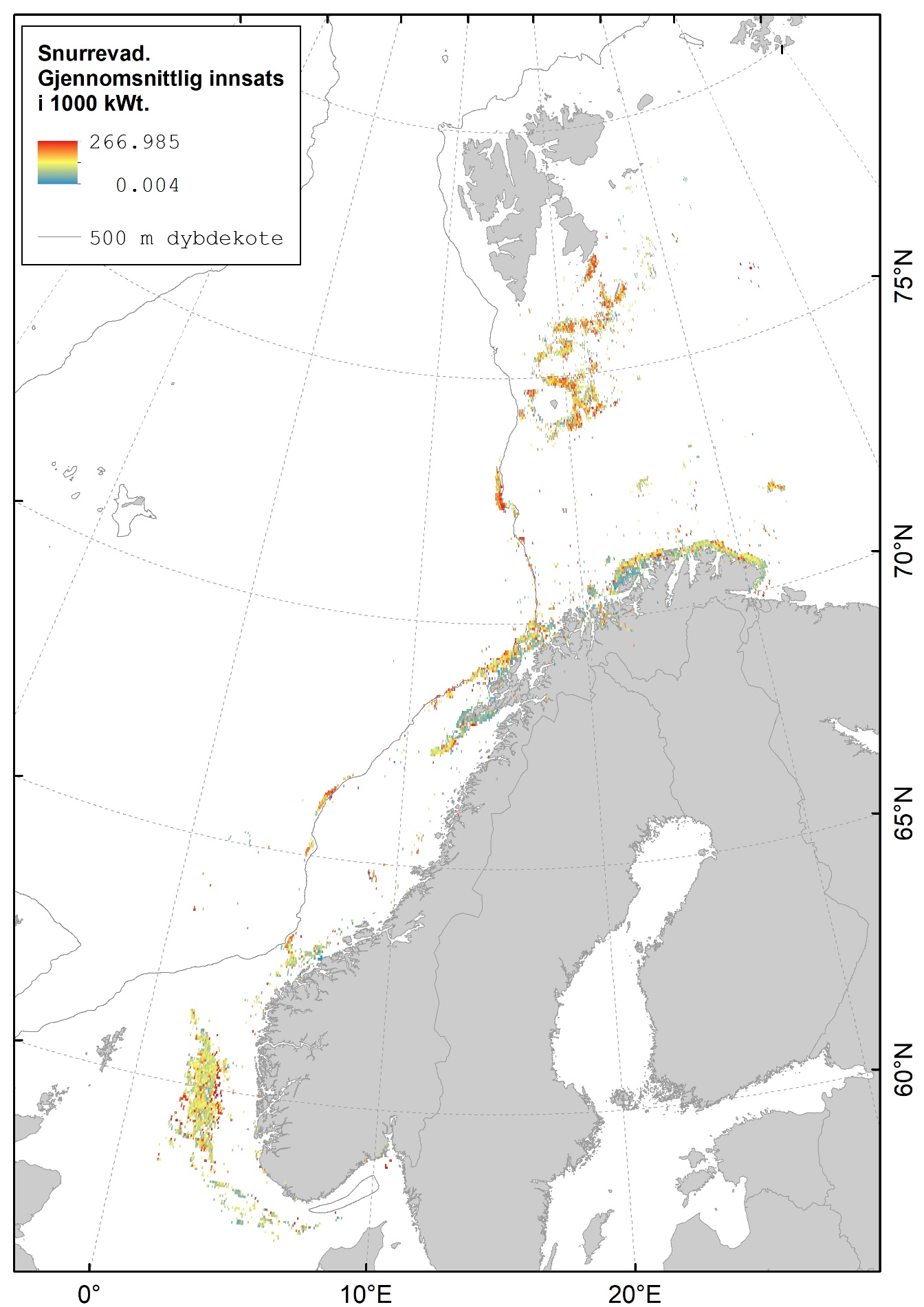
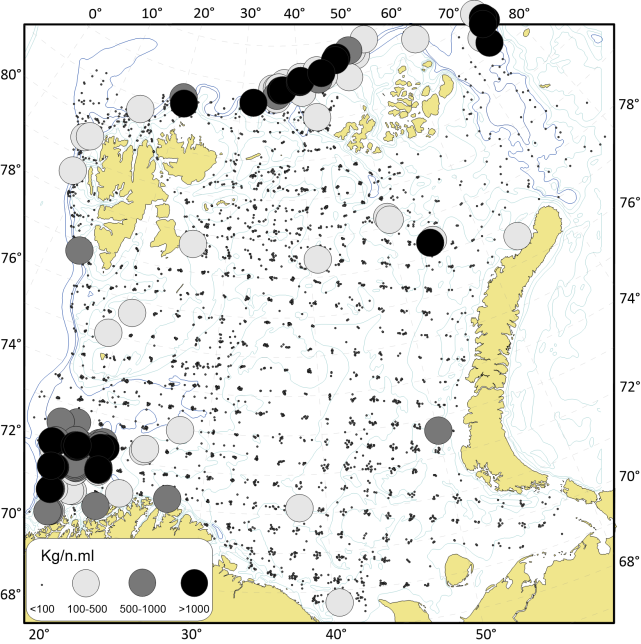
Karta gitt nedenfor viser hvor det fiskes med fisketrål (Figur 2.1), reketrål (Figur 2.2) og snurrevad (Figur 2.3) i norske farvann. Dataene er basert på elektronisk rapportering (ERS-data) til Fiskeridirektoratet sendt fra norske båter over 15 m (https://www.fiskeridir.no/Tall-og-analyse/AApne-data/elektronisk-rapportering-ers). Karta viser gjennomsnittlig innsats (i perioden 2015 til 2021) for hver 0,05 graders (3 arc-minutt) blokker (celler). Enheten for innsats (fiskeintensitet) er gitt som kWt, dvs. kW (mål for båtstørrelse) multiplisert med antall timer (mål for varighet til fangstoperasjonen). Sporingsdata (loggbok-data og VMS-data) kan brukes som en tilnærming til omfanget (utbredelsen) av områder påvirket av fiskeriaktiviteter (Eigaard et al., 2016). Figurene inkluderer ikke fiskeaktivitet fra utenlandske fartøyer som har tilgang til norske farvann.
I Barentshavet finnes de mest intensive fiskeriene med fisketrål i de sørøstlige områdene og langs kysten av Finnmark. Det er også stor fiskeriaktivitet rundt Bjørnøya (spesielt i sør) og sør for Spitsbergen (Figur 2.1). I de sentrale delene av Barentshavet er det tilnærmet ingen trålaktivitet.
I Norskehavet er det størst trålaktivitet på Tromsøflaket, utenfor Vesterålen/Lofoten og sørvestover langs eggakanten. Lengre sør finnes de viktigste trålfiskeriene på Haltenbanken og bankene utenfor Møre.
I Nordsjøen opererer trålflåten langs skråningen av Norskerenna og på sokkelen videre vestover, samt på fiskebankene i sør. Fiskeriaktiviteten i Skagerrak er konsentrert langs Norskerenna.
Reketrålfiskeriet i Barentshavet foregår i hovedsak på Storbanken og Sentralbanken, og i mindre grad i fjordene på Svalbard og sørlige Barentshavet (Figur 2.2). Det fiskes med reketrål i fjordene i Finnmark og Troms og videre sørover til Trøndelag. I Nordsjøen og Skagerrak opererer reketrålerne langs Norskerenna og langs kysten av Rogaland og sørover til Oslofjorden.
Snurrevadfisket i Barentshavet er konsentrert rundt Bjørnøya, videre nordøstover mot Edgeøya og langs hele kysten av Finnmark. I Norskehavet fiskes det med snurrevad på Tromsøflaket og langs eggakanten utenfor Vesterålen og Lofoten. Det er også et snurrevadfiskeri på Mørebankene og på sokkelen vest for Norskerenna i midtre og nordlige deler av Nordsjøen.
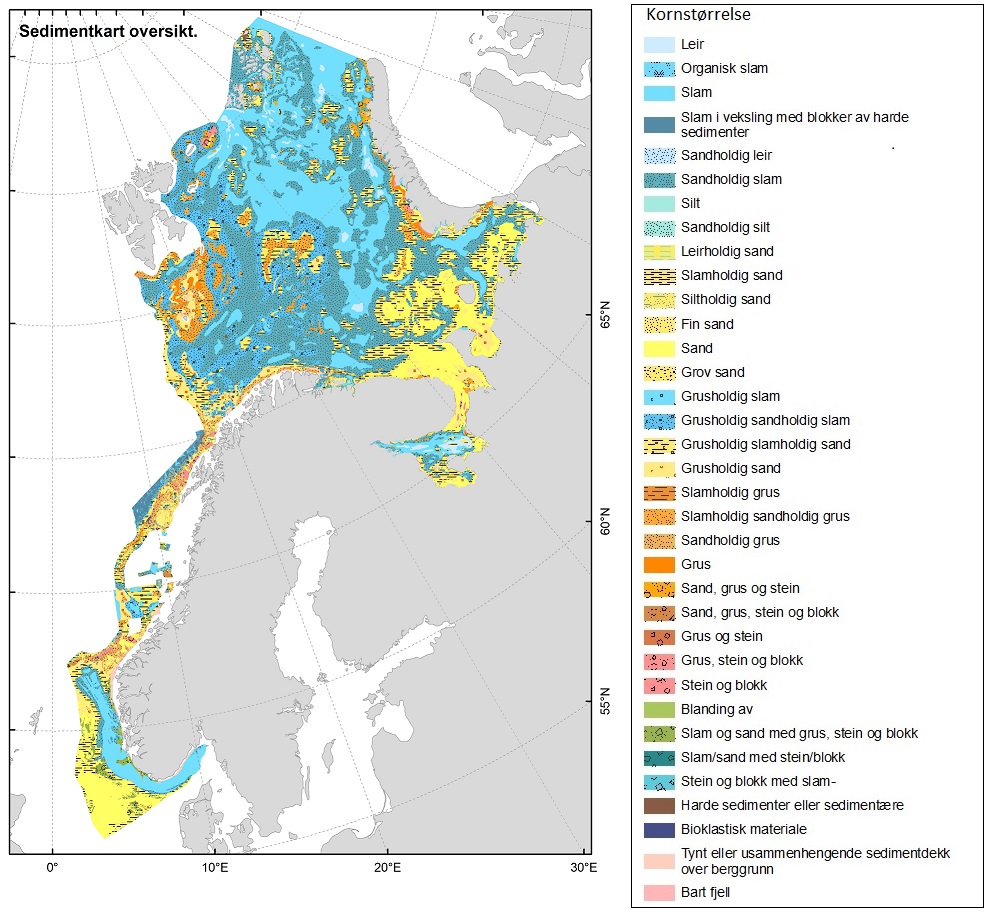
En sammenstilling av sedimentkartet vist i figur 2.4 og karta som viser fiskeriaktiviteten gir en god indikasjon på hvilke bunntyper det tråles mest på i norske farvann. I de områdene av Barentshavet hvor det er størst fiskeriaktivitet (Storbanken, Sentralbanken, Bjørnøya, sør for Spitsbergen) er bunnsedimentene dominert av sand og grus. Bunnsedimentene i de sentrale delene av Barentshavet hvor det er liten fiskeriaktivitet domineres av sandholdig og grusholdig slam. Sørover langs eggakanten er det også overlapp mellom høy fiskeriaktivitet og sand- og grusholdige sedimenter. Fiskeriaktiviteten i Nordsjøen foregår mest på sandbunn, mens det i Skagerrak og langs sørlandskysten i stor grad fiskes på sedimenter bestående av slam.
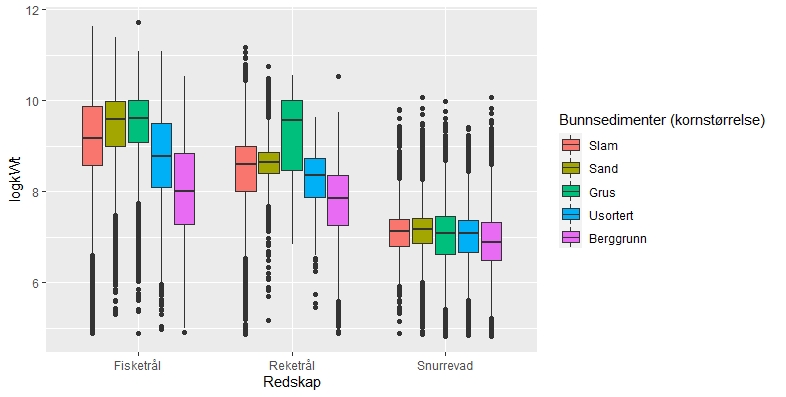
Sammenhengen mellom fiskeriaktivitet og sedimenttype er sammenfattet i figur 2.5. Her framgår det også at fiskeriet med fisketrål er mest utbredt i områder med grus- og sandbunn. Reketrålerne opererer mest på sedimenter bestående av grus og i noe mindre grad på bunn bestående av sand og slam. Snurrevadbåtene fisker i like stor grad på bunn med slam, sand og grus. Berggrunn er den bunntypen hvor det er lavest fiskeriaktivitet.
3 - Bunnfauna i norske farvann
3.1 - Barentshavet og Norskehavet
De store dype områdene i Barentshavet er dekket med slam med innslag av sand, mens de mer strømrike bankene samt kontinentalsokkelen i det sørvestlige Barentshavet for det mest består av sand og grus (Figur 2.4). Analyser gjort på basis av sårbarhet og forekomster av store, fastsittende, sent-voksende og langtidslevende arter gir en god indikasjon på hvilke områder av Barentshavet som er dominert av bunnhabitater som regnes å være sårbare overfor påvirkning fra bunntråling (ICES, 2021).
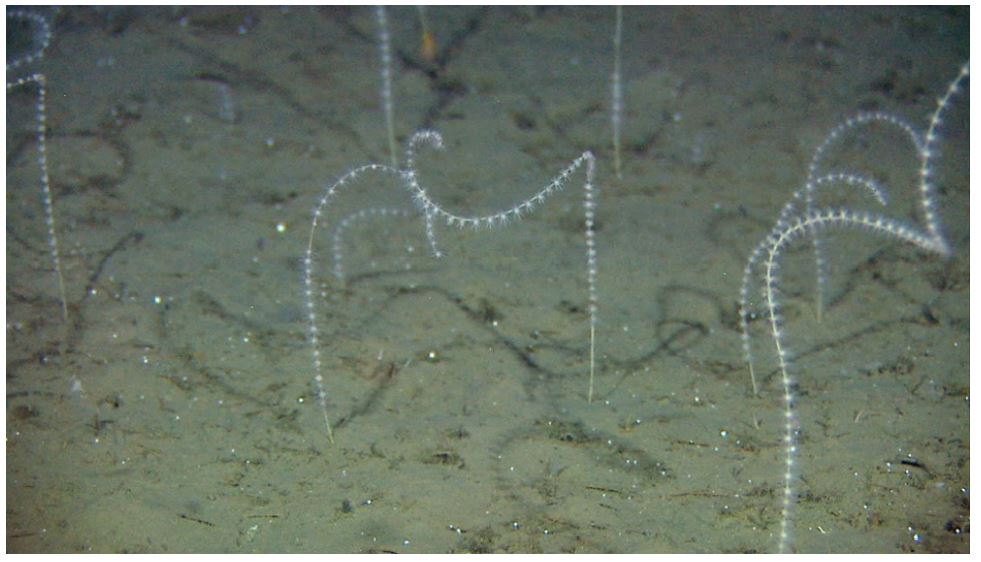
Kartlegging av slike sårbare «indikatorarter» i Barentshavet viser at det i de nordlige arktiske områdene som er dekket av is mye av året, finnes områder med den opptil 2,5 m høye sjøfjær (Umbellula encrinus) som kan bli opptil 70 år gammel (de Moura Neves et al., 2018). Andre sårbare arter i dette området er bløtkoraller (Funiculina, Virgularia, Isidella, Radicipes (Figur 3.1), Paragorgia), blomkålskoraller (Drifa, Duva, Gersemia, Pseudodrifa) og svamper (Figur 3.2). Disse områdene danner strukturelle bunnhabitater med høy biodiversitet (Jørgensen et al., 2019). Deler av det nordlige Barentshavet hvor det historisk har vært lite eller ingen tråling ble i 2019 foreløpig stengt for fiskeri (Jørgensen et al., 2020).
Områdene øst for Svalbard, hvor bunntråling er tillatt, utgjør de delene av Barentshavet som har størst biodiversitet og høyest bunndyrbiomasse. Her finnes store mengder sjøliljer (Heliometra glacialis), blomkålskoraller og medusahoder Gorgonocephalus spp. (opptil 40 cm) som danner morfologisk komplekse samfunn. Disse artene kan ha en levetid på flere tiår. Det nordøstlige Barentshavet har også høy biomasse av store oppreiste og langtidslevende arter (Jørgensen et al., 2020).
I de sørvestlige deler av Barentshavet finnes områder med rike forekomster av Geodia-svamper som kan veie mange kilo og bli en meter i diameter (Certain et al., 2015; Jørgensen et al., 2015). I de samme områdene finnes andre arter av høye og komplekse svamper (Axinella, Phakellia, Haliclona). Bløtbunnsamfunnene i det sørlige Barentshavet består av den lille kop-korallen Caryophylla og bløtkoraller som Flabellum og Isidella (Figur 3.2b).
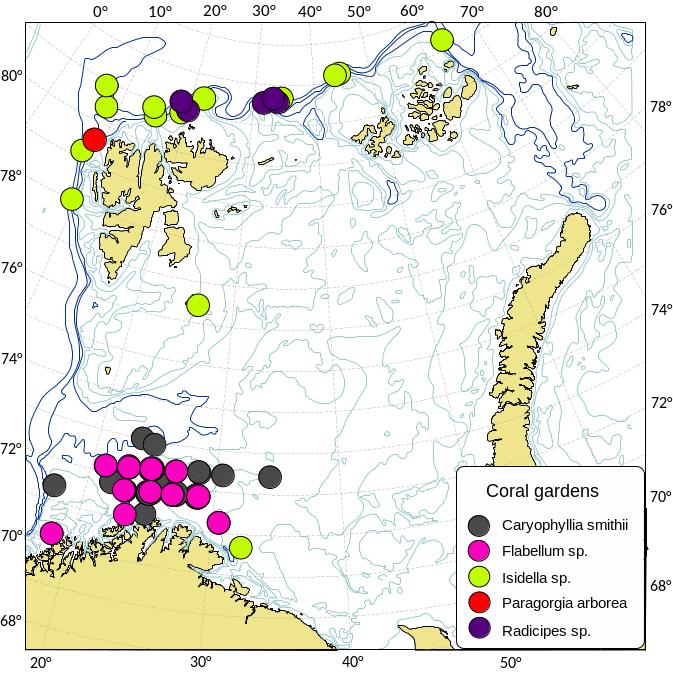
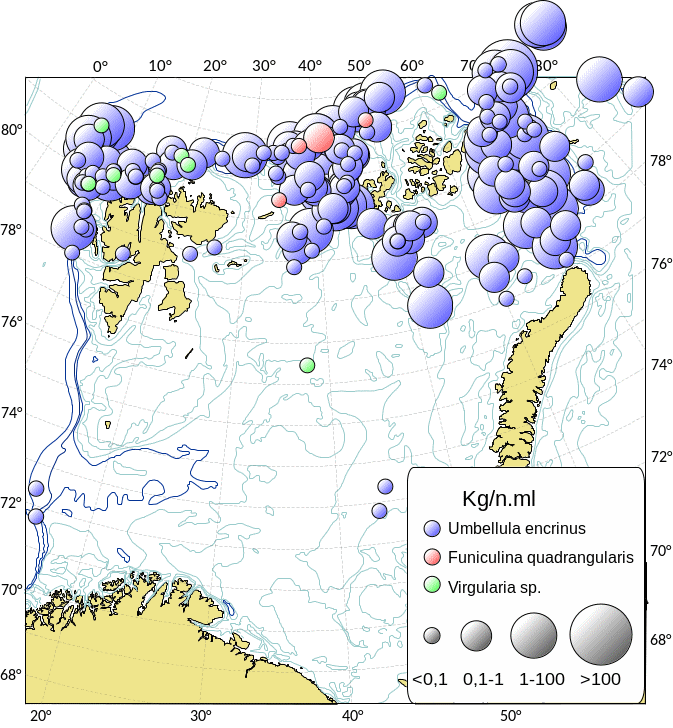
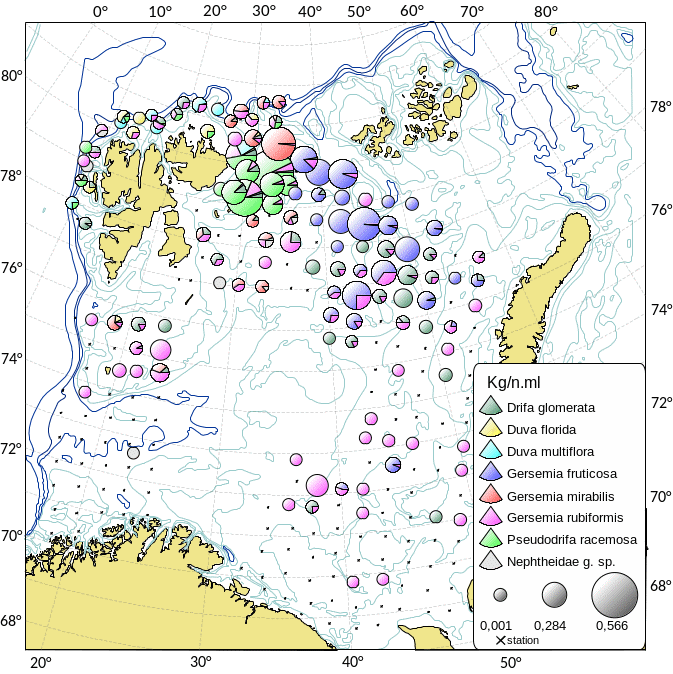
MAREANO programmet har kartlagt Barentshavet og Norskehavet ved hjelp av data fra video og bunnprøver. Denne kartleggingen (MAREANO.no) viser utbredelsen av blomkålskoraller som strekker seg fra lengst nord til lengst sør (lilla i Figur 3.3). Svampspikelbunn (lyserød i Figur 3.3) registret av MAREANO sammenfaller med registeringer fra økosystemtoktet. Slik svampspikelbunn strekker seg også videre sør- og nordover på kontinentalsokkelen i atlantiske vannmasser med mye strøm. Sårbar sjøfjærbunn (gult i Figur 3.3) er utbredt på kontinentalsokkelen.
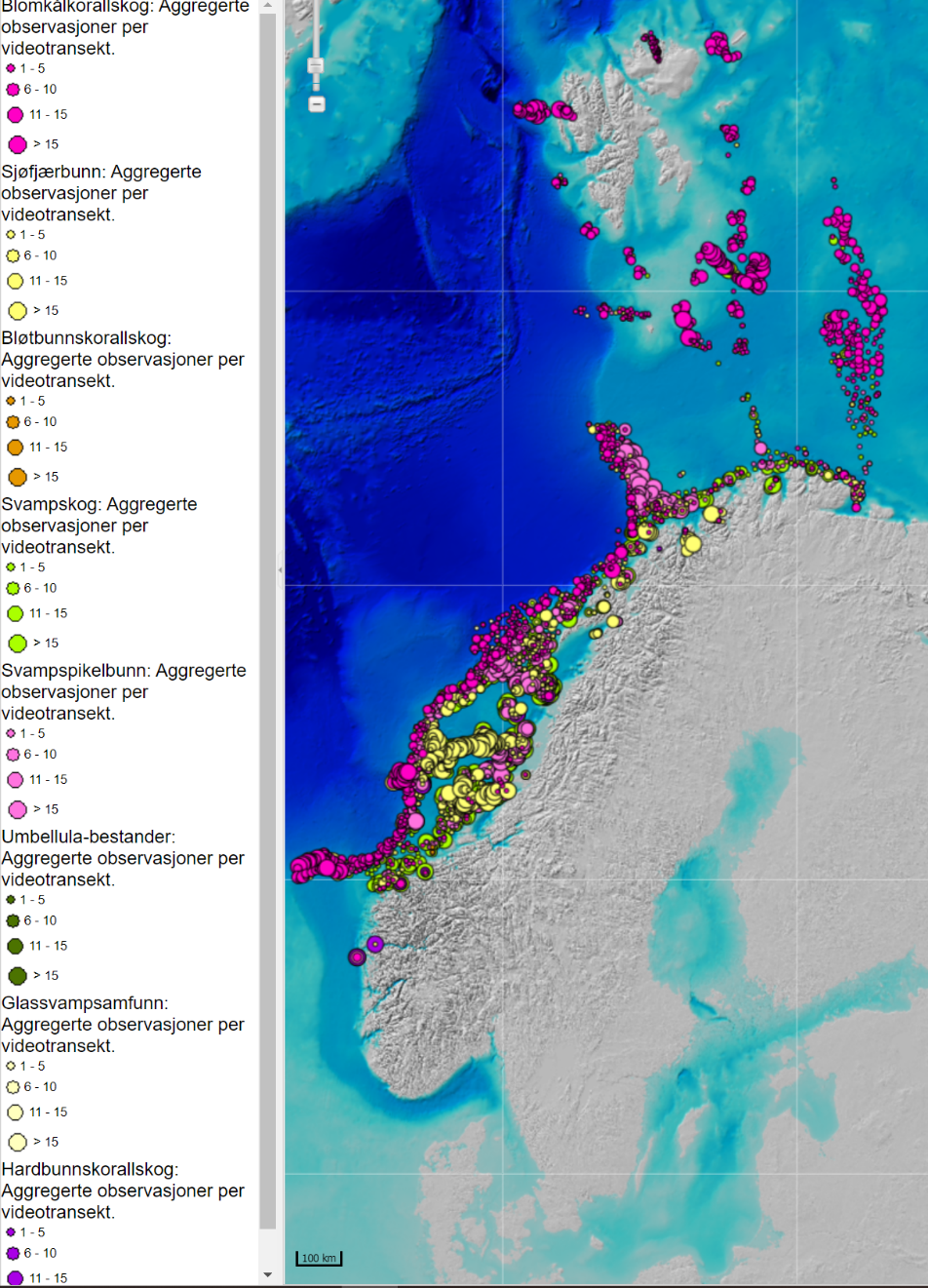
Kaldtvannskorallrev (Desmophylllum pertusum, tidligere kalt Lophelia pertusa) er registrert på 24 lokaliteter i Tromsøflaket/Lopphavet-området. Andre viktige arter i dette området er sjøtreet Paragorgia arborea, den solitære kjøttkorallen Anthomastus grandiflorus og svampspikelbunn med de store Geodia-svampene (Eriksen et al., 2021). Her finnes verdens nordligste korallrev («Korallen», nord for Sørøya) som ble vernet i 1999.
Sokkelen og skråningen utenfor Lofoten og Bjørnøya inneholder flere sårbare naturtyper: dypvannssjøfjær (Umbellula encrinus), grisehalekorallskog (Radicipes gracilis), Lophelia-rev, hardbunnskorallskog og kaldtvanns-svampsamfunn (dominert av glassvamp) (Eriksen et al., 2021). Røstrevet som er ca. 45 km langt er også vernet.
Langs kysten av Norge er det dokumentert 832 korallrev og modellert i overkant av 200 000 korallrev (Eriksen et al., 2021; Mareano.no). Indikatorarter for svampskog (hovedsakelig Axinellide svamper) er hyppig i dette området hvor det også finnes hardbunnskorallskog (Paragorgia, Primnora og Paramuricea). Korallrevene forekommer både kystnært og på kontinentalsokkelen, men er særlig konsentrert til områder langs Storegga og på Sularevet og Iverryggen. Sularevet er et område med rundt 500 individuelle korallrev som forekommer langs en 20-km lang rygg. Disse revene er store (opp til ca. 30 m høye og 500 m lange), gamle (opp mot 9500 år) og i stor grad intakte (bortsett fra på Iverryggen) (Fosså et al., 2015). Med unntak av revene langs Storegga forekommer de fleste korallrevene i sørlige Norskehavet kystnært. Sularevet, Iverryggen og to områder på Storegga (Storneset og Aktivneset) er vernet mot fiske med aktive bunnredskaper.
3.1.1 - Nordsjøen og Skagerrak
Med unntak av Norskerenna er Nordsjøen og Skagerrak karakterisert av grunne områder (2/3 er grunnere enn 100 m, Eriksen et al., 2021). Bunnen domineres av sand, skjellsand og grus i grunne områder og mudder i de dypere delene. Fiskeri med bunntrål er tillatt dypere enn 60 m fra Jærens rev til grensen mot Sverige (se kapittel 1.3.1). Sammenlignende arbeider som har benyttet alle tilgjengelige sporingsdata peker på høy grad av bunntrålpåvirkning i Skagerrak (Eigaard et al., 2017; Kroodsma et al., 2018; Pitcher et al., 2022).
Eriksen et al. (2021) beskriver fire spesielt verdifulle områder i Nordsjøen og Skagerrak: Boknafjorden, tobisfelt spredt over sentrale deler av Nordsjøen, Norskerenna og ytre Oslofjord. Viktigheten til disse områdene er blant annet knyttet til naturverdier og ressurser på bunnen. Bunndyrsamfunnene utgjør viktig føde for torsk, hyse og flyndre. Med unntak av korallrevene i Østfold, er utbredelsen av biogene habitater og habitatbyggende arter lite kjent (Eriksen et al., 2021). Pågående kartlegging gjennom prosjektene Frisk Oslofjord og Mareano vil gi mer kunnskap om koraller, sjøfjær og svamper.
Boknafjorden blir vurdert til høy verdi av Eriksen et al. (2021) blant annet på grunn av bunnsamfunn med store rekeforekomster, svamper i Karmøyfeltet og bambuskorall. Dypere deler er dominert av bløt bunn med innslag av hardbunn i bratte sider og terskler. Det er etablert verneområde for korallrev i Midtsund (Mareano.no) og store svampbestander er kartlagt på Karmøyfeltet.
Tobisfeltene i sentrale Nordsjøen er karakterisert av grus og sandbunn med mye mobil bunnfauna som er viktige gyte- og oppvekstområder for tobis (samlebetegnelse for flere arter sil). Områdene er blant annet viktige for havsil som er rødlistet (Artsdatabanken 2021). Havsil er både kommersielt viktig og i tillegg viktig byttedyr for torskefisk, makrell, sild og flyndrer. Mens de midtre og sørlige områdene har hatt en økning av gytebestandene, er bestandene i de nordlige områdene fortsatt kritisk lave selv etter to tiår med fiskestopp.
Norskerenna er dyprenna (250-700 m) som går parallelt langs kysten fra ytre Oslofjord til Stad. Vannet i Norskerenna er kaldere og saltere enn resten av Nordsjøen/Skagerrak-området og bunnen er dominert av mudder (Eriksen et al., 2021). Norskerenna er overvintringsområde for raudåte og faunaen skiller seg ut som eneste område med dyphavsarter som mesopelagisk fisk, og arter som sjøkreps, dypvannsreke og laksesild. Den har de rikeste forekomstene av bambuskorall registrert i norske farvann og har også høye tettheter av sjøfjær. Det foregår trolig lite bunntråling i de dypeste delene av Norskerenna.
Ytre Oslofjord er preget av høy planktonproduksjon og ulike vannmasser som møtes. Ferskvann fra Glomma som drenerer ut i den ytre delen av fjorden møter nordgående havstrømmer fra Kattegat og Nordsjøen. Bunnen har både undervannsfjellrygger og dype bassenger med bløt bunn. Det dypeste området er Hvalerrenna (450 m) som domineres av slam. Dette er leveområder for dyphavsreke, amfipoder og sjøkreps som får tilført tarerester fra grunt vann (Ramirez-Llodra et al., 2016). I grunnere områder er bunnen variert med sand, grus, stein og fast fjell. Korallrevene (Desmophylllum pertusum) ved Hvaler og Koster er blant verdens største kystnære korallrev (Fosså et al., 2015), og det er påvist hardbunnskorallskog og svampsamfunn i Hvalerdypet (Frisk Oslofjord sluttrapport 2022). Ellers er bunnfaunaen i ytre Oslofjord mangelfullt kartlagt.
4 - Fysiske og kjemiske effekter på bunnsedimenter
4.1 - Fysisk påvirkning av bunntrål og snurrevad

Tråldører, senterklump, sveiper og bunngir er de fire delene av en bunntrål som har bunnkontakt. Disse komponentene vil ha ulike fysisk påvirkning på bunnen både når det gjelder trykket mot bunnen, penetrasjonsdyp og arealet som påvirkes. Det er brukt ulike metoder for å måle penetrasjonsdypet: undervannsvideo og -foto, sidesøkende sonar, sedimentprofiler, markører, dykkere, høyoppløselig akustikk og laser. En sammenfatning av studier av penetrasjonsdypet der disse metodene er anvendt er gitt av Hiddink et al. (2017). Siden det er anvendt forskjellige metoder, er ikke estimatene av penetrasjonsdyp direkte sammenlignbare mellom studiene.
Tråldørene og senterklumpen er de delene av redskapen som er tyngst og som graver dypest ned i sedimentet, men samtidig er dørene og senterklumpen de komponentene som gir det smaleste avtrykket (det minste arealet). Hiddink et al. (2017) gir en oversikt over 18 undersøkelser som viser at tråldørene lager spor som varierer i dybde fra 0,2 til 30 cm (medianverdi = 5,5 cm) der de dypeste sporene er observert på mudderbunn. En av disse studiene er utført i norsk økonomisk sone av Barentshavet (Fiskevernsonen ved Bjørnøya) (Humborstad et al., 2004). Sidesøkende sonar og videokamera blei brukt til å observere trålspor på bunnsedimenter bestående av sand og grus. Tråldørene laget 10 cm dype og 20 cm breie furer og bygde opp en 10 cm høy kant. Observasjoner som var gjort fire måneder senere viste ingen trålspor. En tilsvarende undersøkelse er utført på Grand Banks (Newfoundland, Canada) der det ble trålt på sandbunn (Schwinghamer et al., 1996; 1998). Disse observasjonene viste også at tråldørene lagde furer i sedimentene og bygde opp en kant langs furene. Den fysiske påvirkningen på bunntopografien hadde forsvunnet i løpet av ett år. Begge disse studiene var utført i områder med sterk strøm. Basert på video-observasjoner fra MAREANO er tettheten av trålspor kartlagt langs kysten av Troms og Finnmark. I de undersøkte områdene ble det i snitt observert 1,1 trålspor per 100 m og størst tetthet ble observert på Tromsøflaket med et maksimum på 8,9 (Buhl-Mortensen et al., 2015). Nylig registrerte MAREANO trålspor nord for Svalbard ned til 900 m dyp der det var høyest tetthet av trålspor mellom 200 til 400 m dyp.
En undersøkelse gjort i en beskyttet skotsk fjord viste at trålsporene fortsatt var synlige etter 18 måneder, men sporene var da veldig svake (Tuck et al., 1998). En studie i Biscayabukta som primært undersøkte resuspensjon av bunnsedimenter viste at tråldørene lagde 4-5 cm dype og 20 cm breie spor på mudder/sandbunn (Mengual et al., 2016). En undersøkelse fra Adriaterhavet utført på mudder/sandbunn utmerker seg ved at det ble målt (med sidesøkende sonar) opptil 20-30 cm høye furer etter tråldørene (Lucchetti and Sala, 2012). Andre deler av trålen lagde kun svake spor.
De fleste studiene av fysiske effekter av bunntråling er utført på mudder- og sandbunn. O’Neill et al. (2009) anvendte laserteknologi til å foreta presise målinger på bunnsedimenter bestående av grus. Tråldørene lagde 5-6 cm dype furer og bygde opp en 4-5 cm høy kant. De samme studiene viste at på mudder/sandbunn lagde et lodd på 1,2 tonn en markant fure som var 12 cm dyp. Videoobservasjoner utført på hard steinbunn (dominert av småstein på < 6,5 cm) på kontinentalsokkelen av Alaskabukten viste at bunngiret (gummihjul på 65 cm i diameter) på trålen lagde 1 til 8 cm dype furer og forflytta 19 % av de største steinblokkene (> 75 cm) (Freese et al., 1999).
Alle undersøkelsene av fysiske effekter av bunntråling der det er brukt akustiske (sidesøkende sonar) eller visuelle (kamera) observasjonsmetoder viser spor i bunnsedimentene (se Figur 4.1). Tråldører og senterklump lager de tydeligste og dypeste sporene, mens andre deler av trålen kun lager svake spor. Sveipene er den delen av redskapen som påvirker det største arealet. Den eneste studien som har målt penetrasjonsdyp for sveipene har estimert det til 2,2 cm (Hiddink et al., 2017).
I fiske med snurrevad er det taua og bunngiret som gir bunnkontakt. Taua som kan være over to tusen meter lange, påvirker et langt større areal enn giret. Det finnes ingen studier som har undersøkt hvordan disse komponentene påvirker bunnsedimentene. I en undersøkelse der den fysiske påvirkningen fra ulike mobile bunnredskaper ble modellert, antok forfatterne at penetrasjonsdypet til snurrevadtaua var lik sveipene til en bunntrål (Eigaard et al., 2016). Det kan antas at den fysiske påvirkningen fra snurrevad er mindre enn for trål siden en snurrevad ikke har tråldører og bunngiret har en lettere oppbygning. Modelleringer gjort av Eigaard et al. (2016) indikerte at en snurrevad kun påvirker overflatesedimenter uten fysiske effekter nedover i sedimentlagene. Videre er arealet som påvirkes langt mindre, og siden taua lett kan hekte seg i bunnstrukturer, er det klare begrensninger i bunntypen det fiskes på med snurrevad.
Ved å vekte penetrasjonsdypet til hver av redskapskomponentene (dører, sveiper og bunngir) med bredden på arealet de dekker (henholdsvis en faktor på 0,02, 0,73 og 0,25) har Hiddink et al. (2017) beregnet at en bunntrål i gjennomsnitt graver seg 2,4 cm ned i sedimentet. Studien viste en nær korrelasjon mellom penetrasjonsdyp og mengde bunnfauna som fjernes under tråling. Dette forholdet mente forfatterne forklarer at bomtrål og skjellskraper påvirker bunnhabitater i større grad enn bunntrål. Szostek et al. (2022) gjorde beregninger basert på de samme studiene som i Hiddink et al. (2017) og estimerte penetrasjonsdypet for bunntrål til 1,2 cm.
Varighet av de fysiske endringene i bunnsedimentene vil avhenge av type bunntrål og den opprinnelige dybden til trålsporene, type bunnsediment, strømforhold, bølgeaktivitet og biologisk aktivitet (Tuck et al., 1998; Smith et al., 2000; Humborstad et al., 2004).
4.2 - Oppvirvling av bunnsedimenter
Bunntråling virvler opp sedimenter både som følge av direkte kontakt med havbunnen og på grunn av hydrodynamisk turbulens rundt redskapen. Effekten er størst på bløt mudderbunn bestående av leire og silt. I norske farvann er det gjennomført få studier av denne typen bunnpåvirkning, men ett eksperiment er gjennomført i Eidangerfjorden (Grenlandsområdet) (Bradshaw et al., 2012). Forsøkene viste at et 1,8-km langt trålhal produserte en sky av oppvirvlet sediment på 3-5 millioner m3 som inneholdt 9 tonn silt. Sedimentskyene hadde en bredde på 120–150 m og en høyde på 15-18 m over bunnen. Mesteparten av oppvirvlete (resuspenderte) sedimenter vil synke tilbake til bunnen i løpet av timer, mens lette partikler kan bli værende i vannmassene i flere dager (Durrieu de Madron et al., 2005).
Turbiditeten og den økte sedimenteringen som frembringes av bunntråling på bløte sedimenter kan påvirke marine organismer (Humborstad et al., 2006). Det er få studier av dette og vanskelig å generalisere på tvers av geografi og bunntyper. Økosystemer knyttet til bunnen av fjorder med bløte sedimenter (leire og silt), lav strømhastighet, sjelden utskifting av vannmassene og dypereliggende områder preget av lite naturlig påvirkning vil være mest utsatt for oppvirvling av bunnsedimenter. Tidsserieobservasjoner gjennomført i Middelhavet viste at bunntråling forårsaket en betydelig forflytning av fint sediment fra sokkelen og nedover til større dyp (Puig et al., 2012).
4.3 - Effekter på sedimentære karbonlagre (remineralisering)
Organisk karbon lagret i bunnsedimenter kan konverteres til uorganisk karbon, for eksempel CO2, i en prosess som kalles remineralisering. Dette er komplekse nedbrytningsprosesser som påvirkes av mange faktorer. En inngående beskrivelse av disse prosessene er gitt i Appendiks (engelsk tekst). Her gis en sammenfatning av dette temaet.
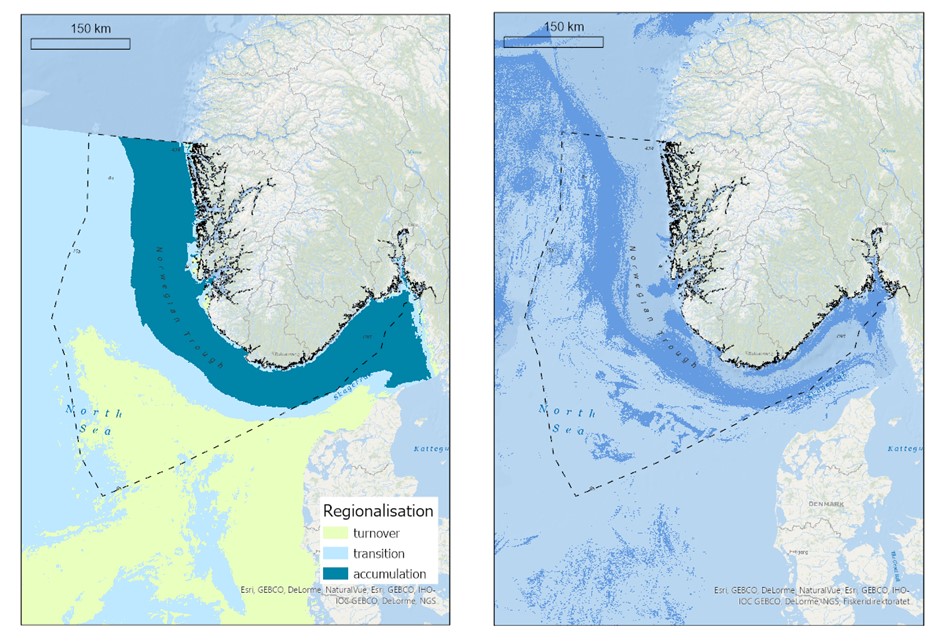
Organisk karbon tilføres havbunnsedimenter gjennom avrenning fra land (elver) og sedimentering av døde marine plante- og dyreorganismer. I norske havområder er det kun gjort beregninger av mengde organisk karbon lagret i Nordsjøen og Skagerrak (Diesing et al., 2021). De høyeste konsentrasjonene av akkumulert organisk karbon ble funnet i Norskerenna, mens det var lave nivåer i store deler av Nordsjøen (Figur 4.2).
Omdannelse av organisk karbon lagret i havbunnen til uorganisk karbon oppløst i vannsøylen (bl.a. CO2 ) kan påvirkes av bunntråling. Noen effekter av tråling vil tendere mot å øke frigivelse av CO2 , mens andre vil virke motsatt. Kvantifisering av den samlede nettoeffekten er derfor vanskelig og vil variere med hvor og hvordan påvirkningen fra trålingen foregår. I 61% av 49 ulike studier fant man ingen netto transport av organisk karbon ut av bunnsedimentene, 29% viste en økt transport, mens de resterende 10% viste en netto akkumulering av sedimentært karbon (Epstein et al., 2022).
Publiserte estimater av mengden frigitt karbon som følge av bunntråling varierer likeledes mye og reflekterer den store usikkerheten og de mange antakelsene og forenklingene assosiert med slike beregninger. For eksempel estimerte Sala et al. (2021) i en oppsiktsvekkende studie at mellom 0,58 og 1,47 milliarder tonn CO2 frigjøres globalt hvert år på grunn av bunntråling. Senere publikasjoner peker på at effekten av bunntråling på karbonlagring i bunnsedimenter er mer kompleks (Epstein et al., 2022; Hilborn and Kaiser, 2022), og organisk karbon er mindre sårbart for fysiske forstyrrelser (Smeaton and Austin, 2022) enn antatt av Sala et al. (2021). Estimatene gitt av Sala et al. (2021) er derfor sannsynligvis for høye.
Nedbrytningshastigheten av organisk karbon bestemmes av interaksjonene mellom sediment (kornstørrelse , mengde og sammensetningen av det organiske karbonet), fysiske parametere (temperatur og oksygeninnhol d), biologi (produksjon og bioturbasjon (spor etter gravende og sediment-spisende organismer)) og hydrologi ( blanding og transport av sedimentet). Dette er komplekse prosesser som er dårlig forstått. Det kan imidlertid postuleres at bunntråling vil ha mindre effekt på remineralisering av organisk karbon i områder med sterk påvirkning av bunnstrømmer og bølger, grovkornete sedimenter, stor oksygeninntrengning i sedimentene og naturlig høye remineraliseringshastigheter av organisk karbon. Motsatt, i områder med lite påvirkning av bunnstrømmer og bølger, høye sedimentasjonsrater, finkornete sedimenter, høyere innhold av organisk karbon og lave nivåer av bioturbasjon, naturlige forstyrrelser og oksidasjon kan det ventes høyere nivåer av remineralisert organisk karbon som følge av bunntråling. Norskerenna er et område der fiskeriaktiviteter overlapper med sonen hvor organisk karbon akkumulerer (Figur 4.2). Mer stedsspesifikke og detaljerte undersøkelser av karbonflukser som respons på bunntråling er nødvendig for å verifisere og kvantifisere frigivelsen av CO2 i norske farvann.
5 - Biologiske effekter på bunnfauna
5.1 - Hvordan dokumentere biologiske effekter av bunntråling?
Effekter av bunntråling på bunnhabitater og bunnfauna har blitt undersøkt ved bruk av to metoder. Med den ene metoden utføres det eksperimentell tråling i et uberørt område og fysiske og biologiske parametere i området sammenlignes før og etter tråling og/eller med et upåvirket kontrollområde. Den andre metoden er basert på historiske data på aktiviteten (innsatsdata) i et kommersielt trålfiskeri, og områder som er sterkt påvirket av det aktuelle fiskeriet sammenlignes med områder som er lite eller ikke upåvirket.
Den førstnevnte metoden (eksperimentell tråling) gir eksakte data på hvor intensivt det undersøkte området er påvirket av tråling og gjør det mulig å studere effekten av ulike grader av påvirkning (Sciberras et al., 2018). Metoden gir også nøyaktig informasjon om posisjonen til det berørte området som sikrer at de biologiske bunnprøvene er tatt fra de områdene som har vært i direkte kontakt med trålredskapen. En forutsetning for pålitelige konklusjoner fra slike studier er at disse prøvene sammenlignes med prøver tatt fra flere kontrollområder (replikater) siden bunnsamfunn viser naturlige variasjoner i antall arter, individer og biomasse (Underwood et al., 1992; Lindegarth et al., 2000). Ulempen med metoden er at eksperimentell tråling er begrenset i tid og rom og studier som anvender denne metoden vil derfor ikke gi et reelt bilde av påvirkninger fra kommersielle tråling der det fiskes gjentatte ganger over store områder og gjennom mange sesonger (Hiddink et al., 2017). I de fleste eksperimentelle studiene har det blitt trålt langs smale korridorer i en relativt kort periode. Effekten av tråling kan da bli påvirket av mobile bunndyr som enten vandrer ut av eller inn i det berørte området, for eksempel åtseldyr som beiter på byttedyr som er blitt skadet eller eksponert som følge av trålen (Kenchington et al., 2006).
Studier basert på historiske data fra kommersiell tråling har ikke disse begrensningene. Bunnprøver tatt fra tradisjonelle fiskefelt vil gjenspeile effektene av kommersiell tråling, men i hvor stor grad det aktuelle området er påvirket av tråling er usikkert fordi eksisterende data på trålintensitet har grov oppløsning (Sciberras et al., 2018). I Nordsjøen for eksempel, er trålaktiviteten innenfor ICES sine statistiske blokker flekkvis fordelt (Rijnsdorp et al., 1998; Jennings et al., 2001; Eigaard et al., 2017). Innenfor blokker som har høy fiskeriaktivitet vil det derfor være områder som er lite påvirket av tråling. Den største svakheten med studier basert på historiske data er at de sjelden har sammenlignbare kontrollområder, dvs. nærliggende områder som ikke har vært påvirket av tråling og som tilsvarer det trålte området med hensyn til dyp, strømforhold, type sedimenter og biologisk diversitet (Hansson et al., 2000; Lindegarth et al., 2000). Bentiske habitater viser ofte store naturlige variasjoner i sammensetning (Kenchington et al., 2006), og sikre konklusjoner om effekten av påvirkning fra bunntråling basert på denne metoden krever også at biologiske parametere i flere påvirkete områder sammenlignes med bunnprøver tatt fra flere tilsvarende kontrollområder (se Lindegarth et al., 2000). Det er utført relativt få studier som oppfyller disse kravene.
En mer inngående og kritisk vurdering av styrken og svakhetene med de to metodene er beskrevet i Løkkeborg (2005). I rapporten konkluderes det med at de fleste studier av biologiske effekter av bunntråling har metodiske svakheter og at resultatene som presenteres må tolkes med forsiktighet. To nylig publiserte meta-analyser, der den ene var basert på kun eksperimentelle studier (Sciberras et al., 2018), mens den andre inkluderte studier basert på begge de to metodene (Hiddink et al., 2017), ga ulike resultater, spesielt med hensyn på restitusjonstid (Sciberras et al., 2018).
5.2 - Effekter av tråling på bunnfauna
Det er utført svært få direkte studier av hvordan norske trålfiskerier påvirker bunnhabitater og ingen studier av effekter av fiske med snurrevad. Det er kjent at bunntråling påvirker bunnfauna og habitater, og det er langtidslevende og store fastsittende bunnorganismer (megafauna) som påvirkes mest ved at de blir fysisk skadet eller fanget i redskapen (Løkkeborg and Fosså, 2011 ; Lyubin et al., 2011 ; Puig et al., 2012 ; Buhl-Mortensen et al., 2013 , 2015; Jørgensen et al., 2016 ).
Kutti et al. (2005) utførte en undersøkelse basert på eksperimentell tråling i Fiskevernsonen rundt Bjørnøya. Dette området har vært stengt for tråling siden 1978 og er derfor godt egnet for å sammenligne et område påvirket av eksperimentell tråling med et upåvirket kontrollområde. Undersøkelsen ble utført på 85-100 m dyp i et område dominert av skjellrester, silt, sand og grus. Biologiske effekter av intensiv tråling (ti trålhal innenfor en 200 m brei korridor) ble undersøkt ved å ta prøver av bunnlevende organismer før og umiddelbart etter trålingen og sammenlignet med tilsvarende prøver tatt i et upåvirket kontrollområde. Tråling førte til resuspensjon av overflatesedimenter og at nedgravde skjell (bivalver) ble liggende eksponert på overflaten av bunnsedimentene. Det ble ikke påvist forandring i antall arter eller diversitet basert på antall, mens diversitet basert på biomasse økte som følge av trålingen. Økt diversitet basert på biomasse skyldtes hovedsakelig redusert biomasse av rur (Balanus balanus) og kråkebolle (Strongylocentrotus droebachiensis), sannsynligvis som følge av bifangst og redusert fangbarhet etter tråling. Blandt de undersøkte artene ble det observert skader hos kråkeboller og bivalver, men bare hos en liten andel av individene (< 4% i 30% av prøvene for kråkeboller og langt lavere for bivalver).
Undersøkelsen konkluderte med at eksperimentell tråling ikke forårsaket store forandringer i bunndyrsamfunnet i denne habitattypen. Dette ble forklart med at eventuelle effekter ikke ble avdekket på grunn av naturlige variasjoner og begrenset antall prøver, eller at dette bunndyrsamfunnet er motstandsdyktig mot tråling fordi området er eksponert for høy grad av naturlige forstyrrelser (f.eks. sterk strøm og temperatursvingninger).
En lignende undersøkelser er utført på Grand Bank i det nordvestlige Atlanterhavet (Prena et al., 1999; Kenchington et al., 2001). Dette er en omfattende 3-årig undersøkelse utført i et område med et bunndyrsamfunn som er representativt for denne delen av Atlanterhavet. Tre områder ble trålt tolv ganger i løpet av fem dager i tre år og sammenlignet med tre kontrollområder. Trålingen forårsaket en 24 % reduksjon i biomassen av mega epifauna som slangestjerner, koraller, kråkebolle og snøkrabbe, mens det var ingen effekt for mollusker (Prena et al., 1999). Reduksjonen i biomasse ble forklart med predasjon fra åtseletere på døde eller skadete organismer og redusert fangbarhet etter resuspensjon av sedimenter som følge av trålingen. Det ble påvist 10 % skade blant kråkeboller. Undersøkelsen indikerte en restitusjonstid på ett år eller kortere.
Prøver tatt med grabb viste at antall arter og total mengde makrofauna ble redusert i løpet av 3-årsperioden i både de trålte områdene og kontrollområdene (Kenchington et al., 2001). Reduksjonen hos et stort antall arter i kontrollområdene ble forklart med at dette bunndyrsamfunnet er dynamisk med naturlige variasjoner over tid. I ett av de tre årene ble det påvist nedgang (25 %) i mengde børstemark (polychaeter) umiddelbart etter tråling, men artene restituerte i løpet av ett år. Forfatterne konkluderte med at naturlige temporære variasjoner (antall arter og individer) i dette bunndyrsamfunnet (makrofauna) er det mest fremtreende ved disse resultatene.
En studie basert på undersøkelser av åtte områder i Nordsjøen viste at tråling og naturlige forstyrrelser har tilsvarende påvirkning på bunndyrsamfunn i grunne og eksponerte habitater (van Denderen et al., 2015). Det er derfor vanskelig å påvise effekter av tråling på bunnhabitater som er eksponert for store naturlige forstyrrelser.
MAREANO har kartlagt sårbare naturtyper som svampsamfunn og korallrev. Store deler av Tromsøflaket, som er et viktig fiskefelt for den norske trålflåten, er undersøkt basert på videotransekter. Disse observasjonene viste skader på store svamper (Geodia og Steletta) som ofte blir liggende langs trålsporene. Kedra et al. (2017) undersøkte effekten av tråling basert på en kombinasjon av video og bunnprøver tatt med skrape/trål. Trålte områder av Tromsøflaket hadde lavere artsrikdom og signifikant mindre epifauna både i antall og biomasse sammenlignet med områder som ikke var trålt. Svamphabitater var mest påvirket, og svamper utgjør 90% av biomassen av benthos på Tromsøflaket (Buhl-Mortensen et al., 2015; Jørgensen et al., 2015). Kedra et al., (2017) presiserer at undersøkelsen er basert på få og små områder upåvirket av tråling (kontrollområder) og at resultatene ikke er entydige.
Analyser av video-opptak fra femten korallrev fordelt på fire områder i det sørlige Barentshavet viste at effekter fra bunntråling var tydeligere på korallrev lengre til havs (Korallen) samliknet med kystnære rev (Lopphavet og vest av Sørøya), der henholdsvis 36,5% og 0,6% av de undersøkte lokalitetene viste tegn på påvirkning (Buhl Mortensen, 2017). De mest synlige tegnene var knuste koraller som var spredt langs bunnen. Lokaliteter påvirket av tråling hadde kolonier av Lophelia og gorgonia-koraller som var omtrent halvparten så høye sammenliknet med upåvirkede lokaliteter. Artsrikdom og tetthet av fisk var høyere i påvirket enn i upåvirket korallrev, mens det var motsatt for evertebrater. I Norge har det vært et generelt forbud mot bunntråling på kjente korallrev siden 1999, og det ble observert tegn på gjenvekst av koraller.
5.3 - Restitusjon av bunndyrsamfunn påvirket av tråling
Et tidligere trålpåvirket område er restituert når bunndyrsamfunnet er gjenoppbygd og dets sammensetningen (antall arter, biomasse, diversitet) ikke er signifikant forskjellig fra tilstøtende upåvirkete områder. Det er ikke gjennomført norske studier på restitusjon av bunndyrsamfunn eller økosystem knyttet til havbunnen etter opphør av kronisk bunntråling. Restitusjonstiden etter påvirkning fra tråling vil variere avhengig av type bunndyrsamfunn der for eksempel et bløtbunnsamfunn vil restitueres langt raskere enn et korallrev eller en svamphage.
En meta-analyse basert på 122 eksperimentelle studier (alle type mobile bunnredskaper) viste at fastsittende og langtidslevende arter som svamp, myke («soft») koraller og skjell (bivalver) hadde mye lengre restitusjonstid (> 3 år) enn mobile arter med kort livshistorie som for eksempel børstemark (< 1 år) (Kaiser et al., 2006; Sciberras et al., 2018). Flesteparten av disse studiene var utført i grunne områder (< 40 m) med høy naturlig påvirkning fra strøm og bølger som favoriserer små rasktvoksende og motstandsdyktige arter. En tilsvarende analyse basert også på historiske data fra kommersiell tråling viste lengre restitusjonstid (1,9 til 6,4 år) (Hiddink et al., 2017). Denne forskjellen ble forklart med at restitusjonstiden vil være kortere i eksperimentelle studier der det tråles innenfor et lite avgrenset område med større grad av innvandring og rekruttering fra nærliggende områder (Sciberras et al., 2018).
For bunndyr som lever nedgravd i sedimentet (infauna) kan restitusjonsprosessen gå så raskt som 1,5 år (Tuck et al., 1998), mens for epibentos som Mollusca, Crustacea, Annelida og Echinodermata kan restitusjonstiden være inntil 3 år (Desprez, 2000; Sarda et al., 2000). Undersøkelser utført på Grand Bank (omtalt ovenfor i kap. 5.2) viste at et bunndyrsamfunn bestående av mega epifauna og makrofauna ble restituert i løpet av ett år (Prena et al., 1999; Kenchington et al., 2001). Langtidslevende og fastsittende megafauna som for eksempel mjuke koraller, svamper og langstilkede sjøfjær kan ha en restitusjonstid på opptil 10 år etter påvirkning (Kaiser et al., 2006 ; Jørgensen et al., 2020).
Mange arter som blir påvirket av bunntrål i norske farvann lever lenge. Dette inkluderer flere arter av sjøanemoner, kongsnegl, irregulære sjøpiggsvin, sjøstjerner og skjell som kan leve opp til 20 år. Andre arter som for eksempel den opp til 2 m lange dyphavssjøfjæren kan bli 70 år, mens korallrev, noen svampearter og sjøliljer kan bli så mye som 100 år (Marlin, 2006). Restitusjonstiden for et bunndyrsamfunn blir bestemt av de artene som har de lengste livsløpene og kan derfor ta inntil 100 år.
I fravær av representative før/etter studier av restitusjon av bunndyrfauna etter langvarig påvirkning fra bunntrål er mye av kunnskapen som finnes basert på modellstudier. Rijnsdorp et al. (2020) brukte ulike metoder i sine modeller for Nordsjøen og fant at restitusjon av bunndyr i form av 90% gjenoppbygging av biomasse kan ventes å ta >10 år på bløtbunn. Store forskjeller i restitusjonstid forklares med stedsspesifikke variasjoner i fiskeintensitet og varighet og hvor motstandsdyktige (robuste) habitatene og organismene er overfor påvirkning (Hiddink et al., 2017).
6 - Kunnskapshull
Effekter av bunntråling på sedimenter, habitater og organismer er komplekse, stedsspesifikke og kan vanskelig predikeres på grunn av artsspesifikke forskjeller i sårbarhet og områdemessige variasjoner i fiskeintensitet (McConnaughey and Syrjala, 2014). De tre vesentligste kunnskapshull for evaluering av tråleffekter i norske farvann er:
- Manglende forståelse av tråleffekter på tvers av ulike bunnhabitater og bunndyrsamfunn
Den faktiske og observerte effekten av bunntråling påvirkes av mange faktorer og er derfor vanskelig å kvantifisere og generalisere med mindre man gjennomfører storskala og langvarige forsøk som er designet for å svare på nettopp dette. Betydningen av sedimentsammensetningen (både når det gjelder uorganiske og organiske komponenter), dyp, temperatur, strømforhold og sammensetningen av infauna og epifauna må avdekkes. Slike forsøk er ikke blitt gjennomført i Norge. At aktive fiskeredskaper som berører bunnen påvirker bunndyrsamfunn og medfører endringer av disse er godt dokumentert, men hvilke konsekvenser dette har for økosystemet har i liten grad vært gjenstand for forskning.
Forståelsen av fysiske prosesser som for eksempel remineralisering av organisk karbon lagret i bunnsedimenter og frigivelse av CO2 er også mangelfull. De nylig publiserte estimatene av effekter av tråling på remineralisering av organisk karbon har mange usikkerhetsmomenter, antakelser og forenklinger. Dette skyldes mangel på områdespesifikk forståelse av de komplekse interaksjonene mellom sedimentet (kornstørrelsen, mengde og stabiliteten til organisk karbon), fysiske forhold (temperatur og oksygeninnhold), biologi (produksjon og bio-turbiditet) og hydrologi (blanding og transport av sedimentet) som avgjør lokalt innhold av organisk karbon og remineraliseringsrater. Siden bunntråling i noen tilfeller kan begrense karbonlagring (Epstein et al., 2022), er det behov for områdespesifikke in situ studier for å øke forståelsen av hvordan lagring av organisk karbon i bunnsedimenter påvirkes av tråling.
- Mangel på kunnskap om motstandsdyktighet og restitusjonstid
Motstandsdyktighet (robusthet) betegner i hvor stor grad et økosystem har evnen til å motstå påførte effekter utenfra. Motstandsevnen vil variere med økosystemets sammensetting av organismer, hvilken bunntype samfunnet finnes på og i hvor stor grad det er påvirket av naturlige forstyrrelser. Restitusjonstiden etter påvirkning fra tråling vil derfor være forskjellig for ulike bunnsamfunn og habitater. Dette er i liten grad undersøkt, og gjennomføring av storskala og stedsspesifikke forvaltningseksperimenter vil være nødvendige for å oppnå god kunnskap om dette.
- Mangel på kunnskap om indirekte effekter på økosystemet
Indirekte effekter av tråling, dvs. hvordan den primære effekten av tråling spres gjennom de ulike populasjonene og funksjonelle gruppene i økosystemet (se Pusceddu et al., 2014), er ikke behandlet eksplisitt i denne rapporten. Deler av denne problematikken adresseres til en viss grad i de repeterende bestandsvurderinger i regi av ICES for fiskebestander og ICES/NAFO for rekebestandene. For full implementering av økosystemtilnærmingen til fiske med bunnredskap trengs det imidlertid kvantifisering av fiskets påvirkning på bunnsamfunn (se Pitcher et al. 2022), og bedre kunnskap om økosystemeffekter generelt. Utvikling av tilstandsindikatorer for bunnpåvirkning er viktig for praktisk implementering i fiskeriforvaltningen i tillegg til bedre forståelse og beskrivelse av hvordan tråleffekter forplanter seg i økosystemet utover den direkte effekten på målarten for fisket.
7 - Litteratur
Bradshaw, C, Tjensvoll, I., Sköld, M., Allan, I.J., Molvaer, J., Magnusson, J., Naes, K. and Nilsson, H.C. (2012). Bottom trawling resuspends sediment and releases bioavailable contaminants in a polluted fjord, Environmental Pollution, 170, 232-241. https://doi.org/10.1016/j.envpol.2012.06.019.
Buhl-Mortensen, P. (2017). Coral reefs in the Southern Barents Sea: habitat description and the effects of bottom fishing. Marine Biology Research, 13(10), 1027-1040.
Buhl-Mortensen, L., Aglen, A., Breen, M., Buhl-Mortensen, P., Ervik, A., Husa, V., et al. (2013). Impacts of Fisheries and Aquaculture on Sediments and Benthic Fauna: Suggestions for New Management Approaches. Fisken og Havet.
Buhl-Mortensen, L., Ellingsen, K.E., Buhl-Mortensen, P., Skaar, K.L. and Gonzales-Mirelis, G. (2015). Trawling disturbance on megabenthos and sediment in the Barents Sea: chronic effects on density, diversity, and composition. ICES Journal of Marine Science. Doi:10.1093/icesjms/fsv200
Certain, G., Jørgensen, L.L., Christel, I., Planque, B. and Vinceny, B. (2015). Mapping the vulnerability of animal community to pressure in marine systems: Disentangling impact types and integrating their effect from the individual to the community level. ICES Journal of Marine Science. Doi:10.1093/icesjms/fsv003
Desprez M. (2000). Physical and biological impact of marine aggregate extraction along the French coast of the Eastern English Channel: short- and long-term post-dredging restoration. ICES Journal of Marine Science, 57,1428-1438
Diesing, M., Thorsnes, T. and Bjarnadottir, L.R. (2021). Organic carbon densities and accumulation rates in surface sediments of the North Sea and Skagerrak. Biogeosciences, 18:2139–2160.
Durrieu de Madron, X., Ferre, B., Le Corre, G., Grenz, C., Conan, P., Pujo-Pay, M., Buscail, R. and Bodiot, O. (2005). Trawling-induced resuspension and dispersal of muddy sediments and dissolved elements in the Gulf of Lion (NW Mediterranean). Continental Shelf Research. 25, 2387-2409.
Eigaard, O. R., Bastardie, F., Breen, M., Dinesen, G. E., Hintzen, N. T., Laffargue, P., Mortensen, L.O. et al. (2016). Estimating seabed pressure from demersal trawls, seines, and dredges based on gear design and dimensions. ICES Journal of Marine Science, doi:10.1093/icesjms/fsv099
Eigaard, O. R., Bastardie, F., Hintzen, N. T., Buhl-Mortensen, L., Buhl-Mortensen., P., Catarino, R., Dinesen, G. E. et al. (2017). The footprint of bottom trawling in European waters: distribution, intensity, and seabed integrity. ICES Journal of Marine Science, doi:10.1093/icesjms/fsw194
Epstein, G., Middelburg, J. J., Hawkins, J. P., Norris, C. R. and Roberts, C. M. (2022). The impact of mobile demersal fishing on carbon storage in seabed sediments. Global Change Biology, 28, 2875– 2894. https://doi.org/10.1111/gcb.16105
Eriksen, E., van der Meeren, G.I., Nilsen, B.M., von Quillfeldt, C.H. og Johnsen, H. (2021). Særlig verdifulle og sårbare områder (SVO) i norske havområder – Miljøverdi. Rapport fra Havforskningen, Nr. 2021-26.
Fosså, J.H., Kutti, T., Buhl-Mortensen, P. og Skjoldal, H.R. (2015). Vurdering av norske korallrev. Rapport fra Havforskningen, Nr. 8-2015.
Freese, L., Auster, P.J., Heifetz, J. and Wing, B.L. (1999). Effects of trawling on seafloor habitat and associated invertebrate taxa in the Gulf of Alaska. Marine Ecology Progress Series, 182, 119-126.
Hansson, M., Lindegarth, M., Valentinsson, D. and Ulmestrand, M. (2000). Effects of shrimp-trawling on abundance of benthic macrofauna in Gullmarsfjorden, Sweden. Marine Ecology Progress Series, 198, 191-201.
He, P., Suuronen, P., Ferro., R.S.T. and Lansley, J. (2021). Classification and illustrated definition of fishing gears. FAO Fisheries and Aquaculture Technical Paper No. 672. Rome, FAO. https://doi.org/10.4060/cb4966en
Hiddink, J.G., Jennings, S., Sciberras, M., Szostek, C.L., Hughes, K.M., Ellis, N., Rijnsdorp, A.D. et al. (2017). Global analysis of depletion and recovery of seabed biota after bottom trawling disturbance. Proceedings of the National Academy of Science USA, 114(31), 8301-8306.
Hilborn, R. and Kaiser, M.J. (2022). A path forward for analysing the impacts of marine protected areas. Nature. https://doi.org/10.1038/s41586-022-04775-1
Humborstad, O.-B., Jørgensen, T. and Grotmol, S. (2006). Exposure of cod Gadus morhua to resuspended sediment: an experimental study of impact of bottom trawling. Marine Ecology Progress Series, 309, 247-254.
Humborstad, O.-B., Nøttestad, L., Løkkeborg, S. and Rapp, H.T. (2004). RoxAnn bottom classification system, sidescan sonar and video-sledge: Spatial resolution and their use in assessing trawling impacts. ICES Journal of Marine Science, 61, 53-63.
ICES. 2021. Working Group on the Integrated Assessments of the Barents Sea (WGIBAR). ICES Scientific Reports. 3:77. 236 pp. https://doi.org/10.17895/ices.pub.8241
ICES. 2022. Working Group on the Integrated Assessments of the Barents Sea (WGIBAR). ICES Scientific Reports. 4:50. 235 pp. http://doi.org/10.17895/ices.pub.20051438
Jennings, S., Pinnegar, J.K., Polunin, N.V.C. and Warr, K.J. (2001). Impacts of trawling disturbance on the trophic structure of benthic invertebrate communities. Marine Ecology Progress Series, 213, 127-142.
Jørgensen, L.L., Bakke, G. and Hoel, A.H. (2020). Responding to global warming: New fisheries management measures in the Arctic. Progress in Oceanography. https://doi.org/10.1016/j.pocean.2020.102423
Jørgensen, L.L., Ljubin, P., Skjoldal, H.R., Ingvaldsen, R.B., Anisimova, N. and Manushin, I. (2015). Distribution of benthic megafauna in the Barents Sea: baseline for an ecosystem approach to management. ICES Journal of Marine Science, 72 (2), 595-613.
Jørgensen, L.L., Planque, B., Thangstad, T.H. and Certain, G. (2016). Vulnerability of megabenthic species to trawling in the Barents Sea . ICES Journal of Marine Science. DOI: 10.1093/icesjms/fsv107 .
Jørgensen, L.L., Primicerio, R., Ingvaldsen, R.B., Fossheim, M., Strelkova, N., Thangstad, T.H., Manushin, I. and Zakharov, D. (2019). Impact of multiple stressors on seabed fauna in a warming Arctic. Marine Ecology Progress Series, 608, 1-12.
Kaiser, M. J. , Clarke, K. D. , Hinz, H. , Austen, M. C. V. , Somerfield, P. J. , and Karakassis, I. ( 2006 ). Global analysis of response and recovery of benthic biota to fishing . Marine Ecology Progress Series, 311 , 1- 14 .
Kędra, M., Renaud, P. E., and Andrade, H. (2017). Epibenthic diversity and productivity on a heavily trawled Barents Sea bank (Tromsøflaket). Oceanologia, 59(2), 93-101.
Kenchington, E.L.R., Gilkinson, K.D., MacIssac, K.C., Bourbonnais-Boyce, C., Kenchington, T.J., Smith, S.J. and Gordon Jr., D.C. (2006). Effects of experimental otter trawling on benthic assemblages on Western Bank, northwest Atlantic Ocean. Journal of Sea Research, 56, 249-270.
Kenchington, E.L.R., Prena, J., Gilkinson, K.D., Gordon Jr., D.C., MacIssac, K., Bourbonnais, C., Schwinghamer, P.J., Rowell, T.W., McKeown, D.L. and Vass, W.P. 2001. Effects of experimental otter trawling on the macrofauna of a sandy bottom ecosystem on the Grand Banks of Newfoundland. Canadian Journal of Fisheries and Aquatic Science, 58, 1043-1057.
Kroodsma, D.A., Mayorga, J., Hochberg, T. et al. (2018). Tracking the global footprint of fisheries. Science, 359,904–908. https://doi.org/10.1126/science.aao5646
Kutti, T., Høisæter, T., Rapp. H.T., Humborstad, O.-B., Løkkeborg, S. and Nøttestad, L. (2005). Immediate effects of experimental otter trawling on a sub-artic benthic assemblage inside the Bear Island Fishery Protection Zone in the Barents Sea. American Fishery Society Symposia, 41: 519-528.
Lindegarth, M., Valentinsson, D., Hansson, M. and Ulmestrand, M. 2000. Interpreting large-scale experiments on effects of trawling on benthic fauna: an empirical test of the potential effects of spatial confounding in experiments without replicated control and trawled areas. Journal of Experimental Marine Biology and Ecology, 245, 155-169.
Lucchetti, A. and Sala, A. (2012). Impact and performance of Mediterranean fishing gear by side-scan sonar technology. Canadian Journal of Fisheries and Aquatic Science, 69, 1806-1816.
Lyubin, P. A., Anisimova, A. A. and Manushin, I. E. (2011). Long-term effects on benthos of the use of bottom fishing gears. In: Jakobsen, T. and Ozhigin, V.K. (Eds.), The Barents Sea. Ecosystem, Resources, Management, 768–775, Tapir Academic Press, Trondheim.
Løkkeborg, S. (2005). Impacts of trawling and scallop dredging on benthic habitats and communities.
FAO Fisheries Technical Paper. No. 472. Rome, FAO. 2005. 58p.
Løkkeborg, S. and Fosså, J.H. (2011). Impacts of bottom trawling on benthic habitats. In: Jakobsen, T. and Ozhigin, V.K. (Eds.), The Barents Sea. Ecosystem, Resources, Management, pp. 760-767, Tapir Academic Press, Trondheim.
MarLIN, 2006. BIOTIC - Biological Traits Information Catalogue. Marine Life Information Network. Plymouth: Marine Biological Association of the United Kingdom. Available from www.marlin.ac.uk/biotic
Martín, J., Puig, P., Masque, P., Palanques, A., & Sanchez-Gomez, A. (2014a). Impact of bottom trawling on deep-sea sediment properties along the flanks of a submarine canyon. PLoS One, 9(8), e104536. https://doi.org/10.1371/journal.pone.0104536
Mazor, T., Pitcher, C.R., Rochester, W., Kaiser, M.J., Hiddink, J.G., Jennings, S., Amoroso, R. et al. (2020). Trawl fishing impacts on the status of seabed fauna in diverse regions of the globe. Fish and Fisheries, 22, 72-86.
McConnaughey, R.A. and Syrjala, S.E. (2014). Short-term effects of bottom trawling and a storm event on soft-bottom benthos in the Bering Sea. ICES Journal of Marine Science, 71, 2469-2483.
Mengual, B., Cayocca, F., Hir, P.L., Draye, R., Laffargue, P., Vincent, B. and Garlan, T. (2016). Influence of bottom trawling on sediment resuspension in the “Grande-Vasiere” area (Bay of Biscay, France. Ocean Dynamics, 66, 1181-1207.
de Moura Neves, B., Edinger, E. and Hayes, V.W. (2018). Morphology and composition of the internal axis in two morphologically contrasting deep-water sea pens (Cnidaria:Octocorallia). Journal of Natural History, 52, 659-685.
Oberle F. K. J., Storlazzi, C. D., & Hanebuth, T. J. J. (2016). What a drag: Quantifying the global impact of chronic bottom trawling on continental shelf sediment. Journal of Marine Systems, 159, 109–119. https://doi.org/10.1016/j.jmarsys.2015.12.007
O`Neill, F.G., Summerbell, K. and Breen, M. (2009). An underwater laser stripe seabed profiler to measure the physical impact of towed gear components on the seabed. Fisheries Research, 99, 234-238.
Pitcher, C.R., Hiddink, J.G., Jennings, S., Collie, J., Parma, A.M., Amoroso, R., Mazor, T., Sciberras, M., McConnaughey, R.A., Rijnsdorp, A.D., Kaiser, M.J., Suuronen, P., Hilborn, R. (2022). Trawl impacts on the relative status of biotic communities of seabed sedimentary habitats in 24 regions worldwide. Proceedings of the National Academy of Science USA. 119(2): e2109449119. https://doi.org/10.1073/pnas.2109449119
Prena, J., Schwinghamer, P., Rowell, T.W., Gordon Jr., D.C., Gilkinson, K.D., Vass, W.P. and McKeown, D.L. 1999. Experimental otter trawling on a sandy bottom ecosystem of the Grand Banks of Newfoundland: analysis of trawl bycatch and effects on epifauna. Marine Ecology Progress Series, 181, 107-124.
Puig, P., Canals, M., Company, J. B., Martin, J., Amblas, D., Lastras, G., Palanques, A. et al. (2012). Ploughing the deep sea floor. Nature, 489, 286-290. https://doi.org/10.1038/nature11410
Pusceddu, A., Bianchelli, S., Martin, J., Puig, P., Palanques, A., Masqué. P. and Danovaro, R. (2014). Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. Proceedings of the National Acadamy of Science USA, 111, 8861-8866 https://doi.org/10.1073/pnas.1405454111
Ramirez-Llodra, E., Rinde, E., Gundersen, H. et al. (2016). A snap shot of the short-term response of crustaceans to macrophyte detritus in the deep Oslofjord. Sci Rep 6, 23800. https://doi.org/10.1038/srep23800
Rijnsdorp, A.D., Buys, A.M., Storbeck, F. and Visser, E.G. (1998). Micro-scale distribution of beam trawl effort in the southern North Sea between 1993 and 1996 in relation to the trawling frequency of the sea bed and the impact on benthic organisms. ICES Journal of Marine Science, 55, 403-419.
Rijnsdorp, A. D., Hiddink, J. G., van Denderen, P. D., Hintzen, N. T., Eigaard, O. R., Valanko, S., Bastardie, F. et al. (2020). Different bottom trawl fisheries have a differential impact on the status of the North Sea seafloor habitats. ICES Journal of Marine Science, 77, 1772-1786.
Sala, E., Mayorga, J., Bradley, D., Cabral, R. B., Atwood, T.B., Auber, A. et al. (2021). Protecting the global ocean for biodiversity, food and climate. Nature, https://doi.org/10.1038/s41586-021-03371-z
Sarda, R., Pinedo, S., Gremare, A. and Taboada, S. (2000). Changes in the dynamics of shallow sandy-bottom assemblages due to sand extraction in the Catalan Western Mediterranean Sea. ICES Journal of Marine Science, 57,1446-1453.
Schwinghamer, P., Guigné, J.Y. and Siu, W.C. (1996). Quantifying the impact of trawling on benthic habitat structure using high resolution acoustics and chaos theory. Canadian Journal of Fisheries and Aquatic Science, 53, 288–296.
Schwinghamer, P., Gordon Jr., D.C., Rowell, T.W., Prena, J., McKeown, D.L., Sonnichsen, G. and Guigné, J.Y. (1998). Effects of experimental otter trawling on surficial sediment properties of a sandy-bottom ecosystem on the Grand Banks of Newfoundland. Conservation Biology, 12, 1215–1222.
Sciberras, M., Hiddink, J.G., Jennings, S., Szostek, C.L., Hughes, K.M., Kneafsey, B., Clarke, L.J. et al. (2018). Responses of benthic fauna to experimental bottom fishing: A global meta-analysis. Fish and Fisheries, 19, 698-715.
Smith, C.J., Papadopoulou, K.N. and Diliberto, S. (2000). Impact of otter trawling on an eastern Mediterranean commercial trawl fishing ground. ICES Journal of Marine Science, 57, 1340-1351.
Smeaton, C., & Austin, W. E. N. (2022). Quality not quantity: Prioritizing the management of sedimentary organic matter across continental shelf seas. Geophysical Research Letters, 49, e2021GL097481. https://doi.org/10.1029/2021GL097481
Szostek, C.L., Hiddink, J.G., Sciberras, M., Shepperson, J.L., Thompson, S., Hormbrey, S., Caveen, A. et al. (2022). A tool to estimate the contribution of fishing gear modifications to reduce benthic impact. Journal of Industrial Ecology. https://doi.org/10.1111/jiec.13366
Tuck, I.D., Hall, S.J., Robertson, M.R., Armstrong, E. and Basford, D.J. (1998). Effects of physical trawling disturbance in a previously unfished sheltered Scottish sea loch.
Marine Ecology Progress Series, 162, 227–242.
Underwood, A.J. 1992. Beyond BACI: the detection of environmental impacts on populations in the real, but variable, world. Journal of Experimental Marine Biology and Ecology, 161, 145-178.
van Denderen, P.D., Bolam, S.G., Hiddink, J.G., Jennings, S., Kenny, A., Rijnsdorp, A.D. and van Kooten, T. (2015). Similar effects of bottom trawling and natural disturbance on composition and function of benthic communities across habitats. Marine Ecology Progress Series, 541, 31-43.
Wainwright, W S. C., & Hopkinson Jr, C. S. (1997). Effects of sediment resuspension on organic matter processing in coastal environments: a simulation model. Journal of Marine Systems, 11(3–4), 353–368.
8 - Appendiks
The remineralization of organic carbon (OC) is defined by recycling processes in which OC, as dead organisms (Asper, 1987) and/or metabolites (Duursma, 1963), are broken-down to smaller molecules until entering the water column as dissolved inorganic carbon (DIC) (Emerson, 2013). The rate of this process may be increased by sediment disturbance from bottom fishing due to the reduced production of flora and fauna, the loss of fine flocculent material, increased sediment resuspension, mixing and transport, and increased oxygen exposure (Epstein et al., 2021). However, some processes such as reduced faunal bioturbation and community respiration, increased off-shelf transport and increases in primary production from the resuspension of nutrients, also induced by bottom trawling activity may lead to a decrease in net OC remineralisation (Epstein et al., 2021). The interaction between both positive and negative feedback mechanisms, makes it challenging to identify the impact of trawling on net OC remineralization and associated increases in DIC which are likely site specific. (Keil, 2017; Snelgrove et al., 2018; LaRowe et al., 2020; Rühl et al., 2020). In a recent review of 49 studies that measured changes in sediment OC associated with bottom fishing, 61% of studies observed no significate effect, 29% showed a decrease in sediment OC and 10% showed an increase in sediment OC (Epstein et al., 2021).
Despite these complexities, it has been estimated that just the uppermost centimetre of sediment may have lost ~0.06 Gt of OC due to historical trawling on global continental slopes (Paradis et al., 2021). Sala et al. (2021) estimate 1.47 Pg (Gt) of aqueous CO2 emissions in the first year after trawling due to OC remineralization with continuous trawling lending to a decline in emissions and the stabilization of values after nine years at about 40% of the initial value. This is equivalent to 0.58 Pg (Gt) per year globally. More locally, on the UK shelf, bottom fishing is estimated to remineralise up to ~0.002 Gt of OC per year, assuming that all resuspended OC is remineralised. (Luisetti et al., 2019). However, there are many uncertainties, assumptions, and simplifications in these estimations (Epstein et al., 2021; Hilborn and Kaiser, 2022), in part, due to a lack of site-specific understanding of the complex interactions that determine rates of remineralisation.
More recent studies have therefore focussed on the potential vulnerability (Black et al., 2022) and the annual cumulative disturbance of sedimentary OC stores (Epstein and Roberts, 2022) without explicitly estimating OC remineralization rates. Although progress has been made in mapping OC sediment stocks in recent years (Seiter et al., 2004; Diesing et al., 2017; Lee et al., 2019; Luisetti et al., 2019; Atwood et al., 2020; Legge et al., 2020; Smeaton et al., 2021), in Norway, only the North Sea and Skagerrak have been mapped to date (Diesing et al., 2021). Estimates of OC remineralization, accumulation and burial rates are even more limited (Berner, 1982; Burdige, 2007; Keil, 2017; Wilkinson et al., 2018; Luisetti et al., 2019; Legge et al., 2020; Diesing et al., 2021). Natural rates of OC remineralization and storage also show large spatial and temporal variability. In general, continental shelf and sublittoral zone sediments in summer show the highest rates of OC remineralization (Middelburg et al., 1996; Tabuchi et al., 2010; Brin et al., 2015; Xue et al., 2015) with rates decreasing at higher latitudes (Fiedler et al., 2016; Bourgeois et al., 2017; Zhao et al., 2018a) and deeper depths (reviewed by Chen et al., 2022). Therefore, levels of OC remineralization in response to bottom disturbance by trawling are likely site (and perhaps seasonally) specific and depend on complex interactions between local sediment (e.g. grain size and OC content and stability), environmental conditions (e.g. temperature and oxygenation), biology (e.g. production and bioturbation), and hydrology (e.g. sediment mixing and transport).
8.1 - Local sediment
Local sediment structure and chemistry will, in part, modulate the effect of bottom trawling on OC remineralization. The local stability of OC in the sediment is dependent on the OC molecular size, structure (Amon and Benner, 1996; Van Kaam-Peters et al., 1998) and functional groups (Deng et al., 2019; Kleber and Lehmann, 2019) as well as mineral-organic associations that may inhibit the decomposing activation of enzymes and microbes (Tietjen and G. Wetzel, 2003; Zimmerman et al., 2004). Organic matter, of which OC is a major constituent, is an umbrella term that encompasses a wide range of different substances (e.g., amino acids, sugars, lipids, and lignin) from marine and terrestrial sources (Burdige, 2007). These individual substances vary in their biogeochemical reactivity or degradability. Organic matter reactivity can be seen as a continuum from easily degradable and short-lived (labile) to hard to degrade and long-lived (refractory) (LaRowe et al., 2020). Marine organic matter (e.g., phytoplankton debris) is typically labile, while organic matter from terrestrial sources (e.g., plant litter and soil organic matter) can be considered refractory. Organic matter in marine sediments is a mixture of organic substances from various sources and with varying reactivities. Highly reactive labile constituents will be remineralized first, followed by less reactive substances (Stumm-Zollinger, 1968). Therefore, the overall reactivity of organic matter is reduced over time (Berner, 1980). Likewise, remineralization rates decrease over time.
The rate of remineralization might also change when refractory organic matter comes into contact with labile organic matter; this is called priming (e.g., Bianchi, 2011). Whilst the drivers controlling priming remain unclear, the process must be regarded as important: Simply measuring the reactivity of OC in marine sediments may not fully capture the vulnerability of stored OC when disturbance mixes refractory OC with a labile fraction (Graves et al., 2022) and thereby alters its reactivity. A recently published meta-analysis concluded that, overall, priming increased remineralization of stable OC with the addition of labile OC (Sanches et al., 2021). Disturbance of sediment OC by bottom trawling and mixing of refractory and labile OC might therefore potentially lead to enhanced remineralization and loss of OC.
The grain size and structure of sediment is also important. Sandy sediments are usually associated with deeper oxygen penetration, advective pore water transport, higher levels of natural disturbance leading to lower OC contents and higher rates of remineralization when compared to muds (Burdige, 2007; Huettel et al., 2014). The limited effects of trawling on OC remineralisation in eutrophic fine sediment and coarse sediments has also been attributed to low baseline OC contents. Indeed, in a recent review, of the 61% of reported studies that show no significant effect of bottom trawling on sediment OC content there was a clear trend toward sandy study sites (Epstein et al., 2021).
However, repeated fishing activity can also affect the structure of sediments particularly in finer sediments in less hydrologically active environments (Kaiser et al., 2002; Trimmer et al., 2005; Martín et al., 2014a; Oberle et al., 2016b). In less hydrologically active depositional environments, the resuspension of finer sediments from deeper layers by trawling may lead to a redeposited surface layer of fine sediments. (Palanques et al., 2014; Oberle et al., 2016b; Tiano et al., 2020). In more hydrologically active environments the resuspension and loss of fine material due to transport can lead to an increase in coarse material towards the surface (Martín et al., 2014a; b; Palanques et al., 2014; Pusceddu et al., 2014; Mengual et al., 2016; Oberle et al., 2016b; Paradis et al., 2021), with changes in the vertical structure affecting the environmental conditions to which sediments are exposed and so rates of remineralisation.
8.2 - Environmental conditions
Local environmental conditions such and temperature and oxygen level to which sediments are exposed determine the rates of OC remineralisation. Sediment microbial communities and their metabolic kinetics are highly influenced by temperature (Nedwell, 1999; Trevathan-Tackett et al., 2018) affecting their ability to degrade OC (Malinverno and Martinez, 2015; Roussel et al., 2015; Zang et al., 2020). Low OC remineralization rates have been, in part, linked to lower temperatures in the deep-sea (Weston and Joye, 2005; D’Hondt et al., 2015) and at higher latitudes (Fiedler et al., 2016; Bourgeois et al., 2017; Zhao et al., 2018a), while increasing temperatures due to climate change have also been linked to increased remineralization (Yamamoto-Kawai et al., 2009; Qi et al., 2020).
Oxygen levels are also critical in determining levels of OC remineralization (Hinojosa et al., 2014; Nierop et al., 2017; Kurian et al., 2020). For example, low oxygen concentrations in northern Pacific sediments have been shown to decrease OC remineralisation (Seiter et al., 2005; Jessen et al., 2017). Increased oxygen levels in the sediments can increase microbial respiration and remineralization activity (Kristensen et al., 1995; Dauwe et al., 2001; Keil, 2017; van de Velde et al., 2018). Natural resuspension and changes to the vertical structure of sediments due to physical disturbance by hydrodynamics (Brodersen et al., 2019) or bioturbation (Aller and Cochran, 2019) increase the depth of oxygen penetration increasing OC remineralisation compared to less disturbed anoxic environments (Glud, 2008; Donis et al., 2016).
Although oxygen penetration and/or sediment oxygen concentrations due to acute physical disturbance, such as bottom fishing activity (Allen and Clarke, 2007; Tiano et al., 2019; De Borger et al., 2021) are often short lived, compared to chronic processes such as bioturbation, with generally fast re-establishment of sediment oxygen gradients, these can vary from the pre-disturbed state. (Allen and Clarke, 2007; Tiano et al., 2019; De Borger et al., 2021). Also, the re-establishment of sediment oxygen gradients and their stability would depend on the intensity of fishing activity in an area. However, even when aerobic respiration is dominant in sediment, remineralization rate can be limited by local sediment conditions such as low OC and nutrient concentration, and low temperatures (0–4 oC) as observed in the deep-sea (Weston and Joye, 2005; D’Hondt et al., 2015).
8.3 - Biology
The effect of bottom trawling on OC storage/remineralisation and net local changes in DIC is greatly dependent on the local community, its function, and sensitivity to trawling pressure.
8.3.1 - Infauna and bioturbation
Benthic fauna is critical to biogeochemical cycling in sediments (Middelburg, 2018; Snelgrove et al., 2018; LaRowe et al., 2020; Rühl et al., 2020). Trawling reduces the abundance and diversity of sessile epifauna and burrowing infauna, decreasing benthic biomass and production (Jennings et al., 2001, 2002; Kaiser et al., 2002; Queirós et al., 2006; Sciberras et al., 2018; Tiano et al., 2020; Tillin et al., 2006). Bioturbation (reworking of sediment particles; Ekdale et al., 1984) and bio-irrigation (reworking of sediment solutes; Meysman et al., 2006) by benthic invertebrates are critical for global nutrient and carbon cycling. These possesses can increase OC remineralisation by changing the vertical structure of the sediment increasing the concentrations of oxygen and other electron acceptors (e.g. nitrate, metal oxides and sulphate), that promote microbial breakdown of OC (Hulthe et al., 1998; Meysman et al., 2006; Arndt et al., 2013; Keil, 2017; Snelgrove et al., 2018; LaRowe et al., 2020).
In addition, the transportation of labile OC from the surface to deeper layers may stimulate the microbial remineralisation of more refractory OC stored in deeper sediment (van Nugteren et al., 2009; Middelburg, 2018). Conversely, the transportation of OC to deeper sediment could increase its chance of burial and long-term storage (van der Molen et al., 2012; Middelburg, 2018; Snelgrove et al., 2018; Rühl et al., 2020; De Borger et al., 2021) although this is shown to be site specific (van Nugteren et al., 2009; Bengtsson et al., 2018; Riekenberg et al., 2020).
In heavily trawled areas, large long-lived burrowing species that have the largest effect on nutrient cycling (Olsgard et al., 2008) are replaced by small-bodied, opportunistic, motile infauna, and larger, highly vagrant, scavenging macrofauna (Jennings et al., 2001; 2002; Kaiser et al., 2002; 2006; Thrush & Dayton, 2002; Tillin et al., 2006). The loss of fauna and flora that stabilize sediments can also lead to increased sediment transport and remineralisation (Roberts, 2007)
8.3.2 - Phytoplankton
Levels of primary production in the water column is a significantly driver of OC content in sediments due to vertical transport of dead material (Seiter et al., 2004; Turner, 2015; Atwood et al., 2020). Primary production may be stimulated by an increase in nutrients entering the water column following sediment disturbance (Fanning et al., 1982; Falcão et al., 2003; de Madron et al., 2005; Polymenakou et al., 2005; Pusceddu et al., 2015). In the Mediterranean, modelling based on trawling experiments has estimated that sediment disturbance by fishing gear could increase local net annual primary production by 15% leading to increased OC settlement. However, this will depend on the hydrodynamic conditions and both the transport of nutrients up to the euphotic zone and transport of dead material to the seabed. It is also possible that decreases in light due increased turbidity from resuspended particles may limit photosynthesis and primary production (Ruffin, 1998; Palanques et al., 2001; Adriano et al., 2005; Cloern et al., 2014; Capuzzo et al., 2015).
8.3.3 - Benthic Algae
Ephemeral macroalgae and microphytobenthos have been shown to recover quickly to trawling disturbance, depending on event frequency (MacIntyre et al., 1996; Ordines et al., 2017). However more sensitive habitats like kelp can take years to recover and coralline algae can require decades to recover (e.g. Dayton et al., 1992; Fragkopoulou et al., 2021). The accumulation rate and stability of sediments, critical OC burial and storage (Middelburg, 2018; LaRowe et al., 2020), is enhanced by benthic micro- and macroalgae (Yallop et al., 1994; Miller et al., 1996; Montserrat et al., 2008). It is possible that in some areas the loss of benthic algae could decrease sediment stability, increased benthic mixing and increased oxygen penetration (even in the absence of repeated trawling) leaded to increased OC remineralisation. In addition to increased DIC realised from OC remineralisation, net increases in local DIC may be confounded by reduced photosynthesis. However, this would depend on the local substrate as many kelp areas in Norway are more associated with rocky bottoms with high rates of sediment transport and low OC accumulation, in addition to being at less risk from trawling.
8.4 - Hydrodynamic activity
The interaction of unidirectional currents and oscillating flows caused by sea surface waves with the seabed shapes the general patterns of sediment distribution across continental shelves. Fine-grained deposits (clays, silts, and muds) tend to accumulate in hydrodynamically quiet settings, e.g. in deep basins, which cannot be reached by wave activity and where current speeds are low. Conversely, coarse-grained deposits (sands and gravels) tend to dominate in hydrodynamically active areas, e.g. in the coastal zone, on shelf banks and the shelf break.
An important parameter that influences the way sediments process OC is permeability, or the resistance to flow of water through the sediment (Bear, 1972). Permeability is loosely correlated with the grain-size of the sediment (Wilson et al., 2008); coarser-grained sediments tend to have higher permeabilities and vice versa. Permeability begins to influence biogeochemical processes in the surface layer of marine sediments when pressure gradients in the benthic environment can drive pore-water flows that transport solutes and small particles more effectively than Brownian molecular motion (Huettel et al., 2014). This threshold is reached when permeability exceeds approximately 10 -12 m 2 (Huettel and Rusch, 2000). In fine-grained sediments with permeabilities below this threshold, solute transport is dominated by molecular diffusion due to existing concentration gradients of solutes and the relatively low hydraulic conductivity of the sediments (Huettel et al., 2003). Diffusion in sediment porewater is a very slow process because the diffusing molecules must follow a tortuous path around the sediment grains (Huettel and Webster, 2001). In coarse-grained, permeable sediments with permeabilities above the threshold, advective processes dominate over diffusion.
Unidirectional and wave orbital water flows interacting with microscale topography (e.g. ripples and biogenic mounds) at the water–sediment interface lead to increased fluid exchange rates compared to exchange by molecular diffusion (Huettel et al., 1996; Precht and Huettel, 2003). Interaction of flows with surface microtopography increases oxygen penetration depths (Huettel and Rusch, 2000). The advective supply of oxygen to the sedimentary microbial community facilitates the effective remineralization of OC filtered into the surface layers of permeable sediment (Huettel et al., 2014). Because of advective porewater flows, permeable sediments may act as biocatalytic filters, notable for their high reaction rates, intense recycling, and extreme spatial and temporal dynamics of biogeochemical processes (Huettel et al., 2003; 2014).
In hydrodynamically quiet, depositional environments trawling may increase OC remineralisation, due to the oxygenation of sediments and redeposition of recently expulsed organic material back to the seabed. (Duplisea et al., 2001; Polymenakou et al., 2005; van de Velde et al., 2018). However, remineralization due to trawling activity may be limited in hydrodynamically active environments due to the removal of fauna and finer surface sediments, low OC due to resuspension and lateral/vertical transportation, and typically coarser sediments associated with deeper oxygen penetration and higher natural remineralisation rates. (Burdige, 2007; Huettel et al., 2014; Pusceddu et al., 2014; Tiano et al., 2019; De Borger et al., 2021; Morys et al., 2021). By comparing disturbance caused by trawling with natural disturbance levels due to hydrodynamics, it might be possible to identify areas where anthropogenic fishing disturbance lies beyond the bounds of natural variability (Diesing et al., 2013).
8.5 - Conclusion
Present estimations of the effect of trawling on OC remineralisation have many uncertainties, assumptions and simplifications. This is due to a lack of site-specific understanding of the complex interactions between local sediment (e.g. grain size and OC content and stability), environmental conditions (e.g. temperature and oxygenation), biology (e.g. production and bioturbation) and hydrology (e.g. sediment mixing and transport) that determine local OC contents and rates of remineralization. However, it may be postulated that hydrodynamically quiet, depositional environments with finer sediments, higher OC contents, and low levels of bioturbation, natural disturbance and oxidation may be expected to show higher levels of OC remineralisation in response to sediment disturbance from bottom trawling. Conversely, in hydrodynamically active environments with coarser sediments, greater oxygen penetration and naturally high OC remineralisation rates, trawling activity may be expected to have a smaller effect on carbon cycling. Presently undisturbed areas in deeper/up-welling or Arctic waters may be particularly sensitive, as increasing temperatures have also been linked to increased remineralization (Qi et al., 2020; Yamamoto-Kawai et al., 2009), depending on OC sediment concentration and if nutrients are limiting. These environments are also predicated to be some of the most sensitive to ocean acidification and so even small local change in seawater DIC and carbonate chemistry may have an impact on ecosystem function. More site specific in situ studies of carbon fluxes in response to trawling activity are needed in Norwegian waters, particularly when considering opening new areas to trawling.
8.6 - References
Adriano, S., Massimiliano, F., Sonia, C., Chiara, F., & Marcomini, A. (2005). Organic carbon changes in the surface sediments of the Venice lagoon. Environment International, 31(7), 1002–1010. https://doi.org/10.1016/j.envint.2005.05.010
Allen, J., & Clarke, K. (2007). Effects of demersal trawling on ecosystem functioning in the North Sea: A modelling study. Marine Ecology Progress Series, 336, 63–75. https://doi.org/10.3354/meps336063
Aller, R.C., Cochran, J.K. (2019). The critical role of Bioturbation for particle dynamics, priming potential, and organic C remineralization in marine sediments: Local and basin scales. Front. Earth Sci. 7, 157.
Amon, R.M.W., Benner, R. (1996). Bacterial utilization of different size classes of dissolved organic matter. Limnol. Oceanogr. 41, 41–51.
Atwood, T. B., Witt, A., Mayorga, J., Hammill, E., & Sala, E. (2020). Global patterns in marine sediment carbon stocks. Frontiers in Marine Science, 7, 165. https://doi.org/10.3389/fmars.2020.00165
Asper, V.L. (1987). Measuring the flux and sinking speed of marine snow aggregates, Deep Sea Research Part A. Oceanographic Research Papers, 34 (1), 1-17. https://doi.org/10.1016/0198-0149(87)90117-8.
Arndt, S., Jørgensen, B. B., LaRowe, D. E., Middelburg, J. J., Pancost, R. D., & Regnier, P. (2013). Quantifying the degradation of organic matter in marine sediments: A review and synthesis. Earth-Science Reviews, 123, 53–86. https://doi.org/10.1016/j.earscirev.2013.02.008
Bear J. (1972). Dynamics of Fluids in Porous Media. New York: Am. Elsevier
Bengtsson, M. M., Attermeyer, K., & Catalán, N. (2018). Interactive effects on organic matter processing from soils to the ocean: Are priming effects relevant in aquatic ecosystems? Hydrobiologia, 822(1), 1–17. https://doi.org/10.1007/s10750-018-3672-2
Berner, R. A. (1982). Burial of organic carbon and pyrite sulfur in the modern ocean; Its geochemical and environmental significance. American Journal of Science, 282(4), 451–473. https://doi.org/10.2475/ajs.282.4.451
Black, K.E., Smeaton, C., Turrell, W.R., Austin, W.E.N. (2022). Assessing the potential vulnerability of sedimentary carbon stores to bottom trawling disturbance within the UK EEZ. Front Mar Sci 9:892892. https://doi:10.3389/fmars.2022.892892
Bianchi, T. S. (2011). The role of terrestrially derived organic carbon in the coastal ocean: A changing paradigm and the priming effect. Proceedings of the National Academy of Sciences, 108(49), 19473–19481. https://doi.org/10.1073/pnas.1017982108
Bourgeois, S., Archambault, P., Witte, U. (2017). Organic matter remineralization in marine sediments: A pan-Arctic synthesis: Pan-Arctic Benthic Remineralization. Global Biogeochem. Cycles 31, 190–213.
Brin, L.D., Giblin, A.E. & Rich, J.J. (2015). Effects of experimental warming and carbon addition on nitrate reduction and respiration in coastal sediments. Biogeochemistry 125, 81–95. https://doi.org/10.1007/s10533-015-0113-4
Brodersen, K.E., Trevathan-Tackett, S.M., Nielsen, D.A., Connolly, R.M., Lovelock, C.E., Atwood, T.B., Macreadie, P.I. (2019). Oxygen consumption and sulfate reduction in Vegetated Coastal habitats: Effects of physical disturbance. Front. Mar. Sci. 6 (14).
Burdige, D. J. (2007). Preservation of organic matter in marine sediments: Controls, mechanisms, and an imbalance in sediment organic carbon budgets? Chemical Reviews, 107(2), 467–485. https://doi.org/10.1021/cr050347q
Capuzzo, E., Stephens, D., Silva, T., Barry, J., & Forster, R. M. (2015). Decrease in water clarity of the southern and central North Sea during the 20th century. Global Change Biology, 21(6), 2206–2214. https://doi.org/10.1111/gcb.12854
Chen, Z., Nie, T., Zhao, X., Li, J., Yang, B., Cui, D., Li, X. (2022). Organic carbon remineralization rate in global marine sediments: A review, Regional Studies in Marine Science, Volume 49, 102112, ISSN 2352-4855, https://doi.org/10.1016/j.rsma.2021.102112.
Cloern, J. E., Foster, S. Q., & Kleckner, A. E. (2014). Phytoplankton primary production in the world's estuarine-coastal ecosystems. Biogeosciences, 11(9), 2477–2501. https://doi.org/10.5194/bg112477-2014
Dauwe, B., Middelburg, J. J., & Herman, P. M. J. (2001). Effect of oxygen on the degradability of organic matter in subtidal and intertidal sediments of the North Sea area. Marine Ecology Progress Series, 215, 13–22. https://doi.org/10.3354/meps215013
De Borger, E., Tiano, J., Braeckman, U., Rijnsdorp, A. D., & Soetaert, K. (2021). Impact of bottom trawling on sediment biogeochemistry: A modelling approach. Biogeosciences, 18, 2539–2557. https://doi.org/10.5194/bg-2020-328
Deng, J., Zhu, W., Zhou, Y., Yin, Y. (2019). Soil organic carbon chemical functional groups under different revegetation types are coupled with changes in the microbial community composition and the functional genes. Forests 10 (240).
D’Hondt, S., Inagaki, F., Zarikian, C.A., Abrams, L.J., Dubois, N., Engelhardt, T., Evans, H., Ferdelman, T., Gribsholt, B., Harris, R.N., Hoppie, B.W., Hyun, J.-H., Kallmeyer, J., Kim, J., Lynch, J.E., McKinley, C.C., Mitsunobu, S., Morono, Y., Murray, R.W., Pockalny, R., Sauvage, J., Shimono, T., Shiraishi, F., Smith, D.C., Smith-Duque, C.E., Spivack, A.J., Steinsbu, B.O., Suzuki, Y., Szpak, M., Toffin, L., Uramoto, G., Yamaguchi, Y.T., Zhang, G., Zhang, X.-H., Ziebis, W. (2015). Presence of oxygen and aerobic communities from sea floor to basement in deep-sea sediments. Nat. Geosci. 8, 299–304.
Diesing, M., Kroger, S., Parker, R., Jenkins, C., Mason, C., & Weston, K. (2017). Predicting the standing stock of organic carbon in surface sediments of the North-West European continental shelf. Biogeochemistry, 135(1), 183–200. https://doi.org/10.1007/s1053 3-017-0310-4
Diesing M, Stephens D, Aldridge, J. (2013). A proposed method for assessing the extent of the seabed significantly affected by demersal fishing in the Greater North Sea. ICES J Mar Sci 70:1085–1096. https://doi.org/10.1093/icesjms/fst066
Diesing, M., Thorsnes, T., & Bjarnadóttir, L. R. (2021). Organic carbon densities and accumulation rates in surface sediments of the North Sea and Skagerrak. Biogeosciences, 18(6), 2139–2160. https://doi.org/10.5194/bg-18-2139-2021
Donis, D., McGinnis, D.F., Holtappels, M., Felden, J., Wenzhoefer, F. (2016). Assessing benthic oxygen fluxes in oligotrophic deep sea sediments (HAUSGARTEN observatory). Deep Sea Res. I: Oceanogr. Res. Pap. 111, 1–10.
Duplisea, D. E., Jennings, S., Malcolm, S. J., Parker, R., & Sivyer, D. B. (2001). Modelling potential impacts of bottom trawl fisheries on soft sediment biogeochemistry in the North Sea. Geochemical Transactions, 2(14), 112. https://doi.org/10.1186/1467-4866-2-112
Durrieu de Madron, X., Ferré, B., Le Corre, G., Grenz, C., Conan, P., PujoPay, M., Buscail, R., & Bodiot, O. (2005). Trawling-induced resuspension and dispersal of muddy sediments and dissolved elements in the Gulf of Lion (NW Mediterranean). Continental Shelf Research, 25(19–20), 2387–2409. https://doi.org/10.1016/j.csr.2005.08.002
Duursma, E.K. (1963). The production of dissolved organic matter in the sea, as related to the primary gross production of organic matter. Netherlands Journal of Sea Research, 2 (1), 85-94. https://doi.org/10.1016/0077-7579(63)90007-3
Ekdale, A., Bromley, R., & Pemberton, S. (1984). Effects of bioturbation on sediment properties. In Ichnology: The use of trace fossils in sedimentology and stratigraphy. Special Publications of SEPM: Society for Sedimentary Geology, USA.
Emerson, S. (2013). In: Sundquist, E.T., Broecker, W.S. (Eds.), Organic Carbon Preservation in Marine Sediments. In: Geophysical Monograph Series, American Geophysical Union, Washington, D.C.,
Nature 607, E1–E2. https://doi.org/10.1038/s41586-022-04775-1
Hinojosa, J.L., Moy, C.M., Stirling, C.H., Wilson, G.S., Eglinton, T.I. (2014). Carbon cycling and burial in New Zealand’s fjords. Geochem. Geophys. Geosyst. 15, 4047–4063.
Huettel, M. and Rusch, A. (2000). Transport and degradation of phytoplankton in permeable sediment, Limnol. Oceanogr., 45, 534–549, https://doi.org/10.4319/lo.2000.45.3.0534.
Huettel, M. and I. T. Webster. (2001). Porewater flow in permeable sediments, p. 144-179. In B. P. Boudreau and B. B. Jørgensen [eds.], The Benthic Boundary Layer. Oxford University Press.
Huettel, M., Berg, P., & Kostka, J. E. (2014). Benthic exchange and biogeochemical cycling in permeable sediments. Annual Review of Marine Science, 6(1), 23–51. https://doi.org/10.1146/annurev-marine-051413-012706
Huettel, M., Røy, H., Precht, E., and Ehrenhauss, S. (2003). Hydrodynamical impact on biogeochemical processes in aquatic sediments, Hydrobiologia, 494, 231–236.
Huettel, M., Ziebis,W., and Forster, S. (1996). Flow-induced uptake of particulate matter in permeable sediments, Limnol. Oceanogr., 41, 309–322, pp. 78–87.
Epstein, G., Middelburg, J. J., Hawkins, J. P., Norris, C. R., & Roberts, C. M. (2022). The impact of mobile demersal fishing on carbon storage in seabed sediments. Global Change Biology, 28, 2875–2894. https://doi.org/10.1111/gcb.16105
Epstein, G., Roberts, C.M. (2022). Identifying priority areas to manage mobile bottom fishing on seabed carbon in the UK. PLOS Clim 1:1–21. https://doi:10.1371/journal.pclm.0000059
Falcão, M., Gaspar, M. B., Caetano, M., Santos, M. N., & Vale, C. (2003). Short-term environmental impact of clam dredging in coastal waters (south of Portugal): Chemical disturbance and subsequent recovery of seabed. Marine Environmental Research, 56(5), 649–664. https://doi.org/10.1016/s0141-1136(03)00069-2
Fanning, K. A., Carder, K. L., & Betzer, P. R. (1982). Sediment resuspension by coastal waters: A potential mechanism for nutrient re-cycling on the ocean's margins. Deep Sea Research Part A. Oceanographic Research Papers, 29(8), 953–965. https://doi.org/10.1016/0198-0149(82)90020-6
Fiedler, B., Grundle, DS., Schutte, F., Karstensen, J., Loscher, C.R., Hauss, H., Wagner, H., Loginova, A., Kiko, R., Silva, P., Tauhua, T., Kortzinger, A. (2016). Oxygen utilization and downward carbon flux in an oxygen-depleted eddy in the eastern tropical North Atlantic. Biogeosciences, 13, 5633–5647, 2016 https://doi.org/10.5194/bg-13-5633-2016
Glud, R.N. (2008). Oxygen dynamics of marine sediments. Marine Biol. Res. 4, 243–289.
Graves C.A., Benson, L., Aldridge, J., Austin, W.E.N., Dal Molin. F., Fonseca, V.G., Hicks, N., Hynes, C., Kröger, S., Lamb, P.D., Mason, C., Powell, C., Smeaton, C., Wexler, S.K., Woulds, C. and Parker, R. (2022). Sedimentary carbon on the continental shelf: Emerging capabilities and research priorities for Blue Carbon. Front. Mar. Sci. 9:926215. https://doi:10.3389/fmars.2022.926215
Hilborn, R., Kaiser, M.J. (2022). A path forward for analysing the impacts of marine protected areas. https://doi.org/10.4319/lo.1996.41.2.0309.
Hulthe, G., Hulth, S., & Hall, P. O. J. (1998). Effect of oxygen on degradation rate of refractory and labile organic matter in continental margin sediments. Geochimica et Cosmochimica Acta, 8, 1319–1328. https://doi.org/10.1016/S0016-7037(98)00044-1
Jennings, S., Dinmore, T. A., Duplisea, D. E., Warr, K. J., & Lancaster, J. E. (2001). Trawling disturbance can modify benthic production processes. Journal of Animal Ecology, 70(3), 459–475. https://doi.org/10.1046/j.1365-2656.2001.00504.x
Jennings, S., Nicholson, M. D., Dinmore, T. A., & Lancaster, J. E. (2002). Effects of chronic trawling disturbance on the production of infaunal communities. Marine Ecology Progress Series, 243, 251–260. https://doi.org/10.3354/meps243251
Jessen, G.L., Lichtschlag, A., Ramette, A., Pantoja, S., Rossel, P.E., Schubert, C.J., Struck, U., Boetius, A. (2017). Hypoxia causes preservation of labile organic matter and changes seafloor microbial community composition (Black Sea). Sci. Adv. 3, e1601897.
Keil, R. (2017). Anthropogenic forcing of carbonate and organic carbon preservation in marine sediments. Annual Review of Marine Science, 9(1), 151–172. https://doi.org/10.1146/annurev-marine-010816-060724
Kaiser, M. J., Clarke, K. R., Hinz, H., Austen, M. C. V., Somerfield, P. J., & Karakassis, I. (2006). Global analysis of response and recovery of benthic biota to fishing. Marine Ecology Progress Series, 311, 1–14. https://doi.org/10.3354/meps311001
Kaiser, M. J., Collie, J. S., Hall, S. J., Jennings, S., & Poiner, I. R. (2002). Modification of marine habitats by trawling activities prognosis and solutions. Fish and Fisheries, 3, 114–136. https://doi.org/10.1046/j.1467-2979.2002.00079.x
Kleber, M., Lehmann, J. (2019). Humic substances extracted by alkali are invalid proxies for the dynamics and functions of organic matter in terrestrial and aquatic ecosystems. J. Environ. Qual. 48, 207–216.
Kristensen, E., Ahmed, S. I., & Devol, A. H. (1995). Aerobic and anaerobic decomposition of organic matter in marine sediment: Which is fastest? Limnology and Oceanography, 40(8), 1430–1437. https://doi.org/10.4319/lo.1995.40.8.1430
Kurian, S., Kessarkar, P.M., Rao, V.Purnachandra., Reshma, K., Sarkar, A., Pattan, J.N., Naqvi, S.W.A. (2020). Controls on organic matter distribution in oxygen minimum zone sediments from the continental slope off western India. J. Mar. Syst. 207, 103118.
LaRowe, D. E., Arndt, S., Bradley, J. A., Estes, E. R., Hoarfrost, A., Lang, S. Q., Lloyd, K. G., Mahmoudi, N., Orsi, W. D., Shah Walter, S. R., Steen, A. D., & Zhao, R. (2020). The fate of organic carbon in marine sediments—New insights from recent data and analysis. Earth Science Reviews, 204, 103146. https://doi.org/10.1016/j.earscirev. 2020.103146
Lee, T. R., Wood, W. T., & Phrampus, B. J. (2019). A machine learning (kNN) approach to predicting global seafloor total organic carbon. Global Biogeochemical Cycles, 33(1), 37–46. https://doi.org/10.1029/2018gb005992
Legge, O., Johnson, M., Hicks, N., Jickells, T., Diesing, M., Aldridge, J., Andrews, J., Artioli, Y., Bakker, D. C. E., Burrows, M. T., Carr, N., Cripps, G., Felgate, S. L., Fernand, L., Greenwood, N., Hartman, S., Kröger, S., Lessin, G., Mahaffey, C., … Williamson, P. (2020). Carbon on the Northwest European Shelf: Contemporary budget and future influences. Frontiers in Marine Science, 7, Article 143. https://doi.org/10.3389/fmars.2020.00143
Luisetti, T., Turner, R. K., Andrews, J. E., Jickells, T. D., Kröger, S., Diesing, M., Paltriguera, L., Johnson, M. T., Parker, E. R., Bakker, D. C. E., & Weston, K. (2019). Quantifying and valuing carbon flows and stores in coastal and shelf ecosystems in the UK. Ecosystem Services, 35, 67–76. https://doi.org/10.1016/j.ecoser.2018.10.013
Malinverno, A., Martinez, E.A. (2015). The effect of temperature on organic carbon degradation in marine sediments. Sci. Rep. 5 (17861).
Martín, J., Puig, P., Masque, P., Palanques, A., & Sanchez-Gomez, A. (2014a). Impact of bottom trawling on deep-sea sediment properties along the flanks of a submarine canyon. PLoS One, 9(8), e104536. https://doi.org/10.1371/journal.pone.0104536
Martín, J., Puig, P., Palanques, A., & Giamportone, A. (2014b). Commercial bottom trawling as a driver of sediment dynamics and deep seascape evolution in the Anthropocene. Anthropocene, 7, 1–15. https://doi.org/10.1016/j.ancene.2015.01.002
Mengual, B., Cayocca, F., Le Hir, P., Draye, R., Laffargue, P., Vincent, B., & Garlan, T. (2016). Influence of bottom trawling on sediment resuspension in the ‘Grande-Vasière’ area (Bay of Biscay, France). Ocean Dynamics, 66(9), 1181–1207. https://doi.org/10.1007/s10236-016-0974-7
Meysman, F. J. R., Middelburg, J. J., & Heip, C. H. R. (2006). Bioturbation: A fresh look at Darwin's last idea. Trends in Ecology & Evolution, 21(12), 688–695. https://doi.org/10.1016/j.tree.2006.08.002
Middelburg, J. J. (2018). Reviews and syntheses: To the bottom of carbon processing at the seafloor. Biogeosciences, 15(2), 413–427. https://doi.org/10.5194/bg-15-413-2018
Morys, C., Brüchert, V., & Bradshaw, C. (2021). Impacts of bottom trawling on benthic biogeochemistry: An experimental field study. Marine Environmental Research, 169, 105384. https://doi.org/10.1016/j.marenvres.2021.105384
Nedwell, D.B. (1999). Effect of low temperature on microbial growth: lowered affinity for substrates limits growth at low temperature. FEMS Microbiol. Ecol. 30, 101–111.
Nierop, K.G.J., Reichart, G.-J., Veld, H., Sinninghe Damsté, J.S. (2017). The influence of oxygen exposure time on the composition of macromolecular organic matter as revealed by surface sediments on the Murray Ridge (Arabian Sea). Geochim. Cosmochim. Acta 206, 40–56.
Oberle, F. K. J., Storlazzi, C. D., & Hanebuth, T. J. J. (2016a). What a drag: Quantifying the global impact of chronic bottom trawling on continental shelf sediment. Journal of Marine Systems, 159, 109–119. https://doi.org/10.1016/j.jmarsys.2015.12.007
Oberle, F. K. J., Swarzenski, P. W., Reddy, C. M., Nelson, R. K., Baasch, B., & Hanebuth, T. J. J. (2016b). Deciphering the lithological consequences of bottom trawling to sedimentary habitats on the shelf. Journal of Marine Systems, 159, 120–131. https://doi.org/10.1016/j. jmarsys.2015.12.008
Olsgard, F., Schaanning, M.T., Widdicombe, S., Kendall, M.A., Austen, M.C. (2008). Effects of bottom trawling on ecosystem functioning, Journal of Experimental Marine Biology and Ecology, 366, (1–2) 123-133, https://doi.org/10.1016/j.jembe.2008.07.036.
Palanques, A., Puig, P., Guillén, J., Demestre, M., & Martín, J. (2014). Effects of bottom trawling on the Ebro continental shelf sedimentary system (NW Mediterranean). Continental Shelf Research, 72, 83–98. https://doi.org/10.1016/j.csr.2013.10.008
Palanques, A., Guillén, J., & Puig, P. (2001). Impact of bottom trawling on water turbidity and muddy sediment of an unfished continental shelf. Limnology and Oceanography, 46(5), 1100–1110. https://doi.org/10.4319/lo.2001.46.5.1100
Paradis, S., Goñi, M., Masqué, P., Durán, R., Arjona-Camas, M., Palanques, A., & Puig, P. (2021). Persistence of biogeochemical alterations of deep-sea sediments by bottom trawling. Geophysical Research Letters, 48(2), e2020GL091279. https://doi.org/10.1029/2020g l091279
Polymenakou, P. N., Pusceddu, A., Tselepides, A., Polychronaki, T., Giannakourou, A., Fiordelmondo, C., Hatziyanni, E., & Danovaro, R. (2005). Benthic microbial abundance and activities in an intensively trawled ecosystem (Thermaikos Gulf, Aegean Sea). Continental Shelf Research, 25(19–20), 2570–2584. https://doi.org/10.1016/j.csr.2005.08.018
Precht, E. and Huettel, M. (2003). Advective pore-water exchange driven by surface gravity waves and its ecological implications, Limnol. Oceanogr., 48, 1674–1684.
Pusceddu, A., Bianchelli, S., & Danovaro, R. (2015). Quantity and biochemical composition of particulate organic matter in a highly trawled area (Thermaikos Gulf, Eastern Mediterranean Sea). Advances in Oceanography and Limnology, 6(1/2), 21–34. https://doi.org/10.4081/aiol.2015.5448
Pusceddu, A., Bianchelli, S., Martin, J., Puig, P., Palanques, A., Masque, P., & Danovaro, R. (2014). Chronic and intensive bottom trawling impairs deep-sea biodiversity and ecosystem functioning. Proceedings of the National Academy of Sciences of the United States of America, 111(24), 8861–8866. https://doi.org/10.1073/pnas.1405454111
Qi, D., Chen, B., Chen, L., Lin, H., Gao, Z., Sun, H., Zhang, Y., Sun, X., Cai, W. (2020). Coastal acidification induced by biogeochemical processes driven by sea-ice melt in the western Arctic ocean. Polar Sci. 23, 100504.
Queirós, A. M., Hiddink, J. G., Kaiser, M. J., & Hinz, H. (2006). Effects of chronic bottom trawling disturbance on benthic biomass, production and size spectra in different habitats. Journal of Experimental Marine Biology and Ecology, 335(1), 91–103. https://doi.org/10.1016/j.jembe.2006.03.001
Ramirez-Llodra, E., Rinde, E., Gundersen, H. Fagerli CW, Fredriksen S, Gitmark JK, Norling K, Walday MG, Norderhaug KM. (2016). A snap shot of the short-term response of crustaceans to macrophyte detritus in the deep Oslofjord. Sci Rep 6, 23800. https://doi.org/10.1038/srep23800
Riekenberg, P. M., Oakes, J. M., & Eyre, B. D. (2020). Shining light on priming in euphotic sediments: Nutrient enrichment stimulates export of stored organic matter. Environmental Science & Technology, 54(18), 11165–11172. https://doi.org/10.1021/acs.est.0c01914
Roberts, C. M. (2007). The unnatural history of the sea. Island Press.
Roussel, E.G., Cragg, B.A., Webster, G., Sass, H., Tang, X., Williams, A.S., Gorra, R., Weightman, A.J., Parkes, R.J. (2015). Complex coupled metabolic and prokaryotic community responses to increasing temperatures in anaerobic marine sediments: critical temperatures and substrate changes. FEMS Microbiol.Ecol. 91, fiv084.
Ruffin, K. K. (1998). The persistence of anthropogenic turbidity plumes in a shallow water estuary. Estuarine, Coastal and Shelf Science, 47(5), 579–592. https://doi.org/10.1006/ecss.1998.0366
Rühl, S., Thompson, C., Queirós, A. M., & Widdicombe, S. (2020). Missing links in the study of solute and particle exchange between the sea floor and water column. ICES Journal of Marine Science, 77(5), 1602– 1616. https://doi.org/10.1093/icesjms/fsaa060
Sala, E., Mayorga, J., Bradley, D., Cabral, R. B., Atwood, T. B., Auber, A., Cheung, W., Costello, C., Ferretti, F., Friedlander, A. M., Gaines, S. D., Garilao, C., Goodell, W., Halpern, B. S., Hinson, A., Kaschner, K., Kesner-Reyes, K., Leprieur, F., McGowan, J., Lubchenco, J. (2021). Protecting the global ocean for biodiversity, food and climate. Nature, 592, 397–402. https://doi.org/10.1038/s41586-021-03371-z
Sanches, L. F., Guenet, B., dos Anjos Cristiano Marino, N., and de Assis Esteves, F. (2021). Exploring the drivers controlling the priming effect and its magnitude in aquatic systems. J. Geophys.ical Res.: Biogeosci. 126, e2020JG006201. https://doi:10.1029/2020JG006201
Sciberras, M., Hiddink, J. G., Jennings, S., Szostek, C. L., Hughes, K. M., Kneafsey, B., Clarke, L. J., Ellis, N., Rijnsdorp, A. D., McConnaughey, R. A., Hilborn, R., Collie, J. S., Pitcher, C. R., Amoroso, R. O., Parma, A. M., Suuronen, P., & Kaiser, M. J. (2018). Response of benthic fauna to experimental bottom fishing: A global meta-analysis. Fish and Fisheries, 19(4), 698–715. https://doi.org/10.1111/faf.12283
Seiter, K., Hensen, C., Schröter, J., & Zabel, M. (2004). Organic carbon content in surface sediments—Defining regional provinces. Deep Sea Research Part I: Oceanographic Research Papers, 51(12), 2001– 2026. https://doi.org/10.1016/j.dsr.2004.06.014
Seiter, K., Hensen, C., Zabel, M. (2005). Benthic carbon mineralization on a global scale. Global Biogeochem. Cycles 19, GB1010.
Smeaton, C., Hunt, C. A., Turrell, W. R., & Austin, W. E. N. (2021). Marine sedimentary carbon stocks of the United Kingdom’s exclusive economic zone. Frontiers in Earth Science, 9, 50. https://doi.org/10.3389/feart.2021.593324
Snelgrove, P. V. R., Soetaert, K., Solan, M., Thrush, S., Wei, C.-L., Danovaro, R., Fulweiler, R. W., Kitazato, H., Ingole, B., Norkko, A., Parkes, R. J., & Volkenborn, N. (2018). Global carbon cycling on a heterogeneous seafloor. Trends in Ecology & Evolution, 33(2), 96– 105. https://doi.org/10.1016/j.tree.2017.11.004
Stumm-Zollinger, E. (1968). Substrate utilization in heterotrophic bacterial communities. J. Water Pollution Ctrl. Fed., 40, 213-229.
Tabuchi, K., Kojima, H. & Fukui, M. (2010). Seasonal Changes in Organic Matter Mineralization in a Sublittoral Sediment and Temperature-Driven Decoupling of Key Processes. Microb Ecol 60, 551–560. https://doi.org/10.1007/s00248-010-9659-9
Thrush, S. F., & Dayton, P. K. (2002). Disturbance to marine benthic habitats by trawling and dredging: Implications for marine biodiversity. Annual Review of Ecology and Systematics, 33(1), 449–473. https://doi.org/10.1146/annurev.ecolsys.33.010802.150515
Tiano, J. C., van der Reijden, K. J., O'Flynn, S., Beauchard, O., van der Ree, S., van der Wees, J., Ysebaert, T., & Soetaert, K. (2020). Experimental bottom trawling finds resilience in large-bodied infauna but vulnerability for epifauna and juveniles in the Frisian Front. Marine Environmental Research, 159, 104964. https://doi.org/10.1016/j.marenvres.2020.104964
Tiano, J. C., Witbaard, R., Bergman, M. J. N., van Rijswijk, P., Tramper, A., van Oevelen, D., & Degraer, S. (2019). Acute impacts of bottom trawl gears on benthic metabolism and nutrient cycling. ICES Journal of Marine Science, 76(6), 1917–1930. https://doi.org/10.1093/icesjms/fsz060
Tietjen, T., G. Wetzel, R. (2003). Extracellular enzyme-clay mineral complexes: Enzyme adsorption, alteration of enzyme activity, and protection from photodegradation. Aquat. Ecol. 37, 331–339.
Tillin, H. M., Hiddink, J. G., Jennings, S., & Kaiser, M. J. (2006). Chronic bottom trawling alters the functional composition of benthic invertebrate communities on a sea-basin scale. Marine Ecology Progress Series, 318, 31–45. https://doi.org/10.3354/meps318031
Trevathan-Tackett, S.M., Thomson, A.C.G., Ralph, P.J., Macreadie, P.I. (2018). Fresh carbon inputs to seagrass sediments induce variable microbial priming responses. Sci. Total Environ. 621, 663–669.
Trimmer, M., Petersen, J., Sivyer, D. B., Mills, C., Young, E., & Parker, E. R. (2005). Impact of long-term benthic trawl disturbance on sediment sorting and biogeochemistry in the southern North Sea. Marine Ecology Progress Series, 298, 79–94. https://doi.org/10.3354/meps2 98079
Turner, J. T. (2015). Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump. Progress in Oceanography, 130, 205–248. https://doi.org/10.1016/j.pocean.2014.08.005
Van der Molen, J., Aldridge, J. N., Coughlan, C., Parker, E. R., Stephens, D., & Ruardij, P. (2012). Modelling marine ecosystem response to climate change and trawling in the North Sea. Biogeochemistry, 113(1–3), 213–236. https://doi.org/10.1007/s10533-012-9763-7
Van de Velde, S., Van Lancker, V., Hidalgo-Martinez, S., Berelson, W. M., & Meysman, F. J. R. (2018). Anthropogenic disturbance keeps the coastal seafloor biogeochemistry in a transient state. Scientific Reports, 8(1), 5582. https://doi.org/10.1038/s41598-018-23925-y
Van Kaam-Peters, H.M.E., Schouten, S., Köster, J., Sinninghe Damstè, J.S. (1998). Controls on the molecular and carbon isotopic composition of organic matter deposited in a Kimmeridgian euxinic shelf sea: evidence for preservation of carbohydrates through sulfurisation. Geochim. Cosmochim. Acta., 62, 3259–3283.
Van Nugteren, P., Moodley, L., Brummer, G.-J., Heip, C. H. R., Herman, P. M. J., & Middelburg, J. J. (2009). Seafloor ecosystem functioning: The importance of organic matter priming. Marine Biology, 156(11), 2277–2287. https://doi.org/10.1007/s00227-009-1255-5
Weston, N.B., Joye, S.B. (2005). Temperature-driven decoupling of key phases of organic matter degradation in marine sediments. Proc. Natl. Acad. Sci. 102, 17036–17040.
Wilkinson, G. M., Besterman, A., Buelo, C., Gephart, J., & Pace, M. L. (2018). A synthesis of modern organic carbon accumulation rates in coastal and aquatic inland ecosystems. Scientific Reports, 8(1), 15736. https://doi.org/10.1038/s41598-018-34126-y
Wilson AM, Huettel M, Klein S. (2008). Grain size and depositional environment as predictors of permeability in coastal marine sands. Estuar Coast Shelf Sci 80:193–199. https://doi.org/10.1016/j.ecss.2008.06.011
Xue, J., Cai, W., Hu, X., Huang, W., Lohrenz, S.E., Gundersen, K. (2015). Temporal variation and stoichiometric ratios of organic matter remineralization in bottom waters of the northern Gulf of Mexico during late spring and summer. J. Geophys. Res. Oceans 120, 8304–8326.
Yamamoto-Kawai, M., McLaughlin, F.A., Carmack, E.C., Nishino, S., Shimada, K. (2009). Aragonite undersaturation in the Arctic ocean: effects of ocean acidification and sea ice melt. Science 326, 1098–1100.
Zang, H., Blagodatskaya, E., Wen, Y., Shi, L., Cheng, F., Chen, H., Zhao, B., Zhang, F., Fan, M., Kuzyakov, Y. (2020). Temperature sensitivity of soil organic matter mineralization decreases with long-term N fertilization: Evidence from four Q10 estimation approaches. Land Degrad. Dev. 31, 683–693.
Zhao, B., Yao, P., Bianchi, T.S., Arellano, A.R., Wang, X., Yang, J., Su, R., Wang, J., Xu, Y., Huang, X., Chen, L., Ye, J., Yu, Z. (2018). The remineralization of sedimentary organic carbon in different sedimentary regimes of the Yellow and East China Seas. Chemical Geology, 495, 104-117. https://doi.org/10.1016/j.chemgeo.2018.08.012.
Zimmerman, A.R., Chorover, J., Goyne, K.W., Brantley, S.L. (2004). Protection of mesopore-adsorbed organic matter from enzymatic degradation. Environ. Sci. Technol. 38, 4542–4548.