Denne rapporten beskriver 2020-generasjonen av triploid laks og dens diploide referansegrupper. Dette inkluderer mer enn totalt 10 millioner fisk fra 16 forskjellige fiskegrupper produsert ved 9 ulike settefiskanlegg og overført til 10 ulike merdanlegg i Nord-Norge. Altså opplevde de forskjellige fiskegruppene ulike kombinasjoner av vannmiljø, sykdomsutbrudd og lusebehandling. Samtidig som dette kompliserer dataanalysen, så styrker det kunnskapen om oppdrettslaksens utfordringer i merd, og om oppdretternes og fiskehelsepersonellets tilknyttede dilemmaer. Dataene viser tydelig at oppdrettere og fiskehelsepersonell kontinuerlig tar vurderinger og balanserer valg basert på produksjonsmål, fiskehelse, fiskevelferd og regelverkskrav. Eksempelvis ved å velge mer risikofylte avlusingsmetoder for antatt robust fisk, og mer skånsomme metoder for antatt sårbar fisk. Et annet eksempel er lengre fastetid før avlusning hos triploid enn diploid fisk. Dataene viser også at både flytting av fisk og avlusing gir risiko for økt dødelighet i ukene etter behandling sammenlignet med ukene før, både for triploid og diploid laks. Spesielt økte risikoen etter termisk og mekanisk behandling, selv om disse fortrinnsvis ble brukt på fisk som ble vurdert som sterke. Lik tidligere generasjoner av triploid laks var også denne generasjonen mer mottakelig for sykdom, og særlig vintersår hos høstutsatt fisk. Fiskevelferdsmotiverte valg av slaktetidspunkt gav kortere produksjonstid i merd hos triploid enn diploid laks. Allikevel hadde triploid laks høyere total dødelighet i løpet av merdperioden enn diploid. De hadde også mer nedklassing ved slakt og høyere økonomisk fôrfaktor. Resultatene viste følgelig at den triploide laksen hadde dårligere fiskehelse og -velferd, og var dårlig økonomi for oppdretter i forhold til diploid laks.
Production, fasting and delousing of triploid and diploid salmon in Northern Norway
— Report for the 2020-generation
Report series:
Rapport fra havforskningen 2023-20
ISSN: 1893-4536
Published: 14.04.2023
Updated: 16.01.2025
Project No.: 14930-04 NRS TRIPWELL, 295200 FASTWELL, 901687 OptiDeLouse
On request by: SalMar AS, Forskningsrådet, FHF
Research group(s):
Dyrevelferd
Subject:
Fiskevelferd
Program:
Fremtidens havbruk
Approved by:
Research Director(s):
Geir Lasse Taranger
Program leader(s):
Robin Ørnsrud
Norsk sammendrag
Summary
This report is an investigation into the 2020 generation of triploid salmon in Northern Norway and their diploid comparators. The trajectory of 16 fish groups comprising more than 10 million fish is described throughout their production cycles. The commercially cultivated fish vary in origin and rearing environment, and experience different disease and treatment events. The acquired dataset provides a uniquely whole description of the aquaculture production cycle but is challenged by the many confounding factors. Rather than make isolated analyses we evaluated the motivations of farmers within the complex system of management regimes, commercial structures, and animal health. We find that farmers and fish health personnel make decisions which balance welfare needs with production goals, but their decisions are constrained and often forced by regulations. Furthermore, a repeated pattern has emerged in which farmers choose to apply riskier handing operations on fish which are perceived to be stronger while reserving gentler operations for those that are perceived to be weaker, which more often are triploid fish. This was also shown in that they typically chose to fast the triploid fish longer than the diploid before delousing operations. The movement of fish between farms and applying delousing treatments increases mortality in the weeks after the operation compared to before, regardless of ploidy. Mortality especially increased after thermal and mechanical treatments even though these were preferentially applied to fish perceived to be stronger. The susceptibility of triploid fish to health problems was demonstrated by higher prevalence of winter ulcers and mortality during the winter for triploid compared to diploids, especially when the fish were transferred to sea that Autumn. Overall, the triploids were also inferior in their economic prospect for the farmer, compared to diploids they had lower product quality at harvest, required more feed per kg produced, and had a higher cumulative mortality by the time of harvest despite being harvested earlier and at lower weight.
16.01.2025: Figure 9 was updated.
1 - Introduction
The salmon aquaculture industry in Norway is the largest of its kind in the world producing 1.6 million tonnes in 2021, 53% of the total global production (FAO-FIGIS, 2021; Norwegian Directorate of Fisheries, 2022a). Despite economic, welfare, disease and environmental challenges the salmon aquaculture industry has continued to grow through technical improvements, improved husbandry practices, breeding, vaccines, and other production innovations (Midtlyng et al., 2011; Torrissen et al., 2011; Kumar & Engle, 2016; Oliveira et al., 2021; Macaulay et al., 2022). The environmental and economic sustainability of the industry is overseen by the government and managed through several agencies which regulate the aquaculture operations and the growth of the industry through the issuing of production licenses (Hersoug et al., 2021; Sønvisen & Vik, 2021). Of particular concern is the environmental challenges posed by aquaculture to wild populations of salmonids, and in 2012 the Ministry of Fisheries and Coastal Affairs suspended the issuing of new production licenses due to the increasing threat of salmon lice and escaped farmed fish (Hersoug et al., 2021; Sønvisen & Vik, 2021).
Shortly after the moratorium was announced, the ministry passed a regulation to issue “green licenses” which would facilitate the continued sustainable growth of salmon farming production (Ministry of Trade, Industry and Fisheries, 2013). In compliance with this regulation, the Norwegian Directorate of Fisheries promulgated 45 new licenses which farmers could apply for through a competitive bidding process. Twenty of the licenses were earmarked to be awarded to commercial operators in the two northernmost regions of Norway, Troms and Finnmark (Norwegian Directorate of Fisheries, 2022b). The applicants for these licenses had to propose a plan which would address and minimize the threat posed by escapes to the wild Atlantic salmon populations. Further award criteria included a lice limit of 0.25 adult females per fish and a maximum allowable application of three medicinal treatments to control lice throughout the production cycle. In the autumn of 2014, NRS Farming AS and Wilsgård Fiskeoppdrett AS were awarded “green licences” for their proposal to farm sterile Atlantic salmon (Salmo salar) in Troms and Finnmark.
Currently, the only commercially available technique for sterilising salmon is to make the eggs triploid. This is done by a process of exposing newly fertilized eggs to high hydrostatic pressure which induces retention of an extra set of maternal chromosomes (Benfey & Sutterlin 1984b; Benfey, 2016; Golpour et al., 2016; Madaro et al., 2021). The triploid individuals produced in this way are not considered to be genetically modified organisms (GMO) and can therefore be used in standard commercial productions of farmed salmon in Norway (Golpour et al., 2016; Borge et al., 2018). Triploid female Atlantic salmon produce a relatively small number of oocytes and do not enter puberty, and although triploid males can fertilize eggs, the embryos die early in development (Benfey & Sutterlin, 1984a; Fjelldal et al., 2014; Benfey, 2016). This inability to produce viable offspring means that farmed triploid salmon cannot threaten wild populations through genetic introgression.
Compared to diploids produced under the same conditions and using the same feed, triploid salmon exhibit higher prevalence of skeletal deformities and ocular cataracts, lower tolerance to higher temperatures and lower oxygen concentrations, and higher rates of mortality (Tiwary et al., 2004; Taylor et al., 2013). Studies have also shown that the nutritional requirements of triploids differ from that of diploids, and that the prevalence of deformities and cataracts in triploids can be reduced through adjusted diets (Fraser et al., 2012; Benfey, 2016; Sambraus et al., 2017a; Sambraus et al., 2018; Madaro et al., 2021; De Fonseka et al., 2022). Based on this knowledge, the Norwegian Food Safety Authorities decided that NRS Farming AS and Wilsgård Fiskeoppdrett AS had to document the health and welfare of their triploid salmon. A collaboration project between the farm companies, the fish health service and the Institute of Marine Research was therefore established to document the health and welfare of triploid salmon produced in the “green licenses” (Stien et al., 2019; Stien et., 2021ab). This report is part of this documentation, and reports on the outcomes of the 2020-generation, from when they were transferred into sea cages until they were harvested.
It has been suggested through several scientific studies that triploid salmon have a reduced immune response to infection compared to diploids (Taylor et al., 2015; Sambraus et al., 2017b; Sambraus et al., 2020). The earlier investigations of the 2014-2019 generations of triploid salmon farmed under the “green licenses” supported that hypothesis. Outbreaks of infections were typically observed earlier and with greater prevalence and severity in groups of triploid salmon than in diploids. Specifically, it was reported that 2 of the 27 diploid fish groups followed were diagnosed with Infectious Salmon Anaemia (ISA) vs. 10 of the 41 triploid groups (Fisher Exact Test, p = 0.07) (Stien et al., 2019,2021ab). This observation prompted a dedicated study to investigate the susceptibility of triploid salmon to ISA, which found an increased susceptibility with 9.4 odds ratio to primary ISA outbreaks in triploid salmon vs. diploid salmon (Aunsmo et al., 2022). This is of special concern because a diagnosis of ISA requires that the Norwegian Food Authorities establish control measurements including a containment area, restrictions on fish movement and often depopulation of affected farms (Aldrin et al., 2021). Although the results by Aunsmo et al. (2022) seem conclusive they were not able to show the same in controlled experiments, and the complexity of their industry data made it difficult to account for unidentified covariates which may confound the causal relationship.
The investigations of the 2014 to 2019 generations of triploid Atlantic salmon found that they were more susceptible to bacterial skin diseases, such as winter-ulcer disease and tenacibaculosis, and suffered higher mortality rates compared to diploids, especially if they were transferred to the sea in the autumn (Stien et al., 2019; Stien et al 2021ab). Thus, triploids may be especially vulnerable to handling and transport potentially causing damages to the epidermis and scale loss, jeopardizing skin health and increasing the risk of secondary infections.
Farmed salmon are handled and crowded when transported between farms and during delousing treatments. Some of the chemical treatments can be administered with minimal handling by concentrating fish within a cage using a tarpaulin, but more typically the delousing methods require that the fish are moved into well-boats with live fish pumps for treatment. Due to the increased resistance of salmon lice to chemical treatments, including hydrogen peroxide, novel non-chemical treatments have been increasingly used for delousing operations in Norway since 2016 (Aaen et al., 2015; Overton et al., 2019; Myhre Jensen et al., 2020). The most used non-chemical treatments remove lice through mechanical mechanisms (brushes, water sprays and turbulence) or with a warm water bath of 20 – 30s at 28-34°C (Brunsvik, 1997; Grøntvedt et al., 2015; Roth, 2016). While all handling operations increase the risk of poor welfare outcomes, the thermal and mechanical treatments have been associated with an increased mortality rate (Overton et al., 2019; Oliveira et al., 2021; Walde et al., 2021; Persson et al., 2022). Prior to handling, fish are typically fasted (feed withdrawal) for hygiene and welfare reasons. Fasting empties the fish guts which avoids extensive amounts of faeces in the handling/transportation water and fasted fish have a lower metabolic rate and oxygen demand. Although not well documented by scientific investigations fasted fish are reported to be calmer and better able to cope with the stress of handling events (Waagbø et al., 2017; Hvas et al., 2021). There is no knowledge about how fasting time possibly interacts with ploidy specific responses to different handling procedures.
Although handling effects were considered in the reports from the 2014-2019 generations (Stien et al., 2019; Stien et al., 2021ab), the reports focused on disease prevalence and production mortality, as all companies in Northern Norway were only delousing using “welfare friendly” chemical treatments at the start of the project. Since the start of the project thermal and mechanical methods have become more commonly used in Northern Norway and are therefore included in this investigation. For the 2020-generation we evaluate the impact of handling events including moving fish between farms and delousing treatments on fish welfare and mortality as it intersects with ploidy and the husbandry decisions of farmers, a previously uninvestigated topic. Farmers and the fish health service make husbandry decisions throughout the production cycle based on how they perceive the status of their fish and those decisions may have an impact on the fish which could confound the reported triploid industry data in previously unexplored ways (Benfey, 2016; Stien et al., 2019; Madaro et al., 2021; Stien et al., 2021ab).For example, Barrett et al. (2022) suggest that farmers choose to expose apparently strong fish populations to more risky handling, and to slaughter weak fish populations earlier. It is therefore our suspicion, that comparing production mortality between ploidy can give a skewed description of production performance, and that economic feed conversion ratio and harvest quality must also be considered, this was not done in the reports by Stien et al. (2019; 2021ab).
Using production data collected from the “green licenses” at NRS Farming AS and Wilsgård Fiskeoppdrett AS in Finnmark and Troms for the 2020 generation, the main aims of the present report were to investigate:
- Effects on fish health and welfare from handling events as:
- Sea transfer
- Moving fish to a new farm
- Delousing
- If fasting modulates the welfare outcome of delousing
- Differences in wound healing and ulcer development between diploid and triploid
- If the causal relationship between ploidy and ISA remains
- How farmers’ decision affects the results
- Differences in production performance between ploidy
2 - Materials and methods
2.1 - Gathering data from commercial sources
This investigation follows the production of diploid and triploid Atlantic salmon transferred to sea in 2020 at commercial farms in Troms and Finnmark. The proprietary data was gathered through the cooperation with two different aquaculture firms and includes information on the origin and production course of the fish, daily production data for each cage, assessment of external injuries of a subsample of 20 fish done as part of the weekly lice counts by the farmers, health status as recorded by veterinarians at their monthly visits, key data for delousing treatments and harvest data for the production calculated at harvest. Monthly meetings were held between researchers from the Institute of Marine Research, fish health personnel and representatives of the companies to discuss, validate and to explain major events and decisions such as delousing treatments and movement of fish between farms and cages. The data is extensive but because it is commercially derived no controls ensured direct comparability between groups of fish which may differ in their origin, strain, feed, environment, pathogen exposure, disease treatment, and delousing treatment. The priority of farm managers and fish health personnel throughout the production cycle was to achieve the best possible fish health and welfare and the highest quality and quantity of end-product possible, and thus they adapted their husbandry to any posed threat. Here, that framework of understanding was central to the analysis of the data and to the interpretation of the results.
2.2 - Production data
The production data from the companies consists of daily recordings of key production metrics from each cage at each farm, including number of dead fish collected, number of fish euthanised, average sea temperature at three meters depth, and amount of feed distributed to the cage. Additional production data variables are calculated from those metrics. For example, the number of fish in the cage is calculated from the number of fish added at transfer subtracted by number of fish that have died, been euthanized, escaped, or removed from the cage. Inaccuracies in these estimates can for instance result from the error margin of the fish count provided by the delivery boat and the unobserved loss of any fish that is not found in the collector system of dead fish. The average weight of the caged fish is calculated recursively based on the initial average size of the fish at transfer, amount of feed distributed to the fish each day and the respective sea temperature. However, during the production the counts can sometimes be corrected after counting of fish passing through delousing systems, and the weights corrected based on sampling of fish from the respective cages.
2.3 - External injury data
Norwegian salmon farmers are obliged by regulation (Anon, 2012) to sample at least 10 to 20 fish (depending on season) and count the number of adult female, mobile and sessile salmon lice (Lepeophtheirus salmonis) on each of these fish. As part of the project, these fish were also inspected for external injuries. This included scoring of deformities (opercula, spine, upper and lower jaw), skin welfare indicators (fin wound, skin bleeding, snout wound and wound/ulcer on the fish body) and eye welfare indicators (eye clouding and eye bleeding/injury). As far as possible, these data were recorded weekly for each cage at each farm. In short, the farmers inspected each fish, and scored them from 0 to 3, where 0 implies no injury/defect, 1 denotes minor or very mild, 2 is clear, and 3 represents severe injury/defect. In order to facilitate the recordings, the companies commissioned the development of a software program (Røkter 2.0) that saves and produces graphical reports and enables downloading of these data. The scoring system was developed in collaboration with the LAKSVEL-project and includes images of each indicator and scoring level (Nilsson et al., 2022a). In addition to salmon lice, the farmers counted the number of Caligus elongatus (known in Norway as “the Scottish louse”) as these can also pose a welfare threat.
2.4 - Health information
All the farms were visited monthly by the fish health service. A fish health biologist or veterinarian surveyed the sea cages for visible sick or damaged fish at the surface, inspected the dead fish collected that day, and (if deemed necessary) took samples for further testing and detection of pathogens. Based on these observations the fish health service made a monthly report of their general impression of the health situation on the farms and the results of any diagnostic tests of fish from sea cages with suspected problems. These reports are a mixture of qualitative and clinical data, and their exact format and what is included varies. Nonetheless, they provide important insight into the health situation at each farm throughout the productions.
2.5 - Fish groups
|
Group |
Hatc. |
Strain |
T/D |
Farm |
Period |
# |
Weight (g) |
Fish (#) |
|
L1T |
Ha1 |
AG QTL-Innova Shield |
T |
L1 |
7–23 May |
5 |
104 –133 |
145–191 000 |
|
L2T |
Ha2 |
AG QTL-Innova Gain |
T |
L2 |
19 –29 May |
4 |
105 –241 |
115– 182 000 |
|
L3D |
Ha3 |
AquaGen |
D |
L3 |
20 May –11 Jun |
4 |
72 –116 |
138–199 000 |
|
L3T |
Ha4 |
Stofnfiskur |
T |
L3 |
27 May - 1 Aug. |
4 |
98 –107 |
118–200 000 |
|
L2D1 |
Ha5 |
AG QTL-Innova Gain |
D |
L2 |
3–6 June |
2 |
104 –109 |
198–200 000 |
|
L4D |
Ha6 |
SB Salmoprotect |
D |
L4 |
8–24 June |
4 |
78 –107 |
199–201 000 |
|
L2D2 |
Ha7 |
MOWI QTL-IPN |
D |
L2 |
18 June |
1 |
129 |
200 000 |
|
L5D |
Ha8 |
AG QTL-Innova Gain |
D |
L5a |
13 –14 July |
4 |
164 –196 |
197–200 000 |
|
L6T1 |
Ha2 |
SF Salmoselect |
T |
L6a |
27 July –7 Sep. |
4 |
94 –137 |
144–197 000 |
|
L6T2 |
Ha2 |
SB Salmoprotect |
T |
L6a |
31 July |
3 |
102 –131 |
142–198 000 |
|
L7D |
Ha7 |
MOWI QTL-IPN |
D |
L7 |
29 Aug.–18 Oct. |
6 |
100 –126 |
184–200 000 |
|
L8T |
Ha2 |
SB Salmoprotect |
T |
L8a |
26 Sep. – 8 Oct. |
6 |
106 –122 |
199–200 000 |
|
L9D |
Ha9 |
Rauma |
D |
L9 |
28 Sep. |
2 |
128 –138 |
191–200 000 |
|
L9T1 |
Ha4 |
AquaGen |
T |
L9 |
2 Oct. – 14 Nov. |
3 |
104 –115 |
98–194 000 |
|
L10T |
Ha1 |
SB Salmoprotect |
T |
L10 |
13 Oct. –1 Nov. |
4 |
106 –125 |
129–169 000 |
|
L9T2 |
Ha2 |
Stofnfiskur |
T |
L9 |
24 Oct. |
1 |
120 |
147 000 |
The monitoring of the 2020-generation includes 10 million fish that were divisible into 16 distinct fish groups characterized by 8 cultivated strains of Atlantic salmon originating from 9 different hatcheries and transferred to 57 sea cages at 10 different farms (Table 1). Each farm (also referred to as locality or farm site) includes 4 to 8 cages (or net pens) that can each legally hold up to 200 000 fish at 25 kg/m3, and as the fish grows it may be necessary to move and split fish into additional cages. Following corporate structures, the farm may be connected to multiple hatcheries and adjacent farms which can be sources of smolts or fish split from a full pen. It is therefore common for a farm to be comprised of triploid or diploid fish from a single strain but different fish groups can occur at the same locality. At three farms there were both diploid and triploid groups, but these were comprised of fish of different strains from different hatcheries (L2, L3 and L9, Table 1).
2.6 - Moving fish between farms events
Three of the farms moved fish from the original farm to another nearby farm (Table 2). These events exemplify the impacts on fish due to handling and transport, without added stress from delousing treatments. Note that the moved fish were typically split into two cages and in some cases also mixed (Table 2).
|
Group |
From |
# |
Weight (kg) |
Fish (x1000) |
Date |
FD |
°C |
To |
# |
|
L5D |
L5a |
4 |
2.4-2.7 |
177-182 |
Jun-21 |
6 |
6.4 |
L5b |
8 |
|
L6T1 |
L6a |
1 |
1.7-1.7 |
138 |
May-21 |
6 |
4.1 |
L6b |
2 |
|
L6T2 |
L6a |
2 |
1.5-1.6 |
137-166 |
May-21 |
6 |
4.1 |
L6b |
4 |
|
L6T2 |
L6a |
1 |
1.5 |
191 |
Jun-21 |
7 |
9.0 |
L6b |
2 |
|
L8T |
L8a |
6 |
0.9-1 |
176-188 |
Jun-21 |
5 |
9.0 |
L8b |
6 |
2.7 - Delousing events
There were 14 separate chemical delousing events. Two of these were so called mosaic delousings where some of the cages at the farm were treated with AlphaMax Ò (active ingredient deltamethrin), while others were treated with Salmosan Ò (active ingredient azamethiphos). The chemical treatments were typical done using a tarpauline encapsulating the sea cage being treated and there was no changing of cage or splitting in relation to any of the chemical treatments. In addition to treatments by Salmosan and AlphaMax, there was one freshwater treatment, one Hydrogen peroxide (H2O2) treatment and one treatment by Ectosan Vet Ò (active ingredient imidakloprid). The basic properties of the treatment events are listed in Table 3.
|
N. |
Groups |
Farm |
Method |
# |
Weight (kg) |
Fish (x1000) |
Date |
FD |
C |
Tarp |
Dose |
Time |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
1 |
L3D,L3T |
L3 |
H2O2 |
8 |
0.5-1.1 |
114-193 |
Dec 20 |
6-9 |
5.9 |
Y |
|
30-32 |
|
|
2 |
L1T |
L1 |
AlphaMax |
3 |
1.7-1.8 |
141-146 |
Jan-21 |
5-6 |
5.4 |
Y |
150 |
30-33 |
|
|
2 |
L1T |
L1 |
Salmosan |
2 |
1.7-1.8 |
181-182 |
Jan-21 |
5 |
5.4 |
Y |
100 |
60 |
|
|
3 |
L5D |
L5b |
AlphaMax |
7 |
5.2-5.5 |
83-86 |
Nov-21 |
1-2 |
7.0 |
Y |
150 |
30-31 |
|
|
4 |
L8T |
L8b |
AlphaMax |
6 |
2.4-2.7 |
94-128 |
Oct-21 |
1-5 |
9.4 |
Y |
150 |
33.5-35 |
|
|
5 |
L8T |
L8b |
Salmosan |
6 |
3.3-3.5 |
94-127 |
Nov-21 |
6 |
5.5 |
Y |
100 |
||
|
6 |
L6T1 |
L6a |
Salmosan |
5 |
2.3-3.2 |
83-91 |
Sep-21 |
0-2 |
8.4 |
Y |
100 |
60-180 |
|
|
7 |
L6T2,L6T1 |
L6b |
Salmosan |
9 |
3.1-4.0 |
67-94 |
Oct-21 |
3-8 |
8.4 |
Y |
100 |
180 |
|
|
8 |
L6T1 |
L6a |
AlphaMax |
4 |
3.2-4.4 |
82-90 |
Dec-21 |
3 |
6.0 |
Y |
150 |
|
|
|
9 |
L6T2,L6T1 |
L6b |
AlphaMax |
8 |
4.0-5.1 |
65-93 |
Dec-21 |
2-3 |
5.6 |
Y |
150 |
|
|
|
10 |
L9D,L9T1,L9T2 |
L9 |
AlphaMax |
4 |
2.3-3.9 |
59-92 |
Dec-21 |
2-6 |
5.5 |
Y |
150 |
40 |
|
|
10 |
L9D,L9T2 |
L9 |
Salmosan |
4 |
3.0-4.0 |
59-90 |
Dec-21 |
2-3 |
5.5 |
Y |
100 |
180 |
|
|
11 |
L3D,L3T |
L3 |
AlphaMax |
5 |
3.8-5.0 |
69-105 |
Nov-21 |
5-6 |
6.0 |
Y |
150 |
40 |
|
|
12 |
L10T |
L10 |
AlphaMax |
4 |
2.0-2.1 |
113-139 |
Nov-21 |
4-5 |
5.0 |
N |
167 |
30 |
|
|
13 |
L10T |
L10 |
Fresh water |
4 |
2.4-2.5 |
112-137 |
Jan-22 |
7-9 |
3.7 |
N |
|||
|
14 |
L10T |
L10 |
Ectosan vet. |
4 |
2.7-3.0 |
104-126 |
Mar-22 |
5 |
2.9 |
N |
100 |
60 |
|
There were 13 separate delousing events with thermal and 6 separate events with mechanical delousing. All six of the mechanical events were with HydrolicerÒ, eleven of the thermal were with ThermolicerÒ and two with OptilicerÒ, of which one was terminated before completion. The basic properties of the thermal and mechanical treatment events are listed in Table 4 and 5, respectively.
|
N. |
Groups |
Farm |
Method |
# |
Weight (kg) |
Fish (x1000) |
Date |
FD |
°C |
T°C |
DT |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
15 |
L4D |
L4 |
Optilicer |
4 |
2.3-2.7 |
186-188 |
Sep-21 |
6-7 |
8.8 |
|
|
|
16 |
L2T |
L2 |
Thermolicer |
2 |
2.0-2.4 |
100-175 |
Dec-20 |
6-6 |
5.9 |
28 |
22.1 |
|
17 |
L2T, L2D1 |
L2 |
Thermolicer |
3 |
3.7-4.1 |
132-192 |
Sep-21 |
9-13 |
8.7 |
|
|
|
18 |
L5D |
L5a |
Thermolicer |
4 |
1.1-1.3 |
190-195 |
Dec-20 |
3-4 |
7 |
30.5-30.7 |
23.5-3.7 |
|
19 |
L5D |
L5b |
Thermolicer |
8 |
3.7-4.1 |
88-90 |
Aug-21 |
3-4 |
10.5 |
31.2 |
20.7 |
|
20 |
L6T1 |
L6a |
Optilicer* |
1 |
2.9 |
91 |
Sep-21 |
3-3 |
9.1 |
|
|
|
21 |
L9D, L9T1-2 |
L9 |
Thermolicer |
4 (8) |
1.4-2.0 |
123-194 |
Sep-21 |
3-3 |
9.9 |
32.2 |
22.3 |
|
22 |
L9D, L9T1-2 |
L9 |
Thermolicer |
8 |
2.0-3.6 |
59-93 |
Nov-21 |
5-6 |
12 |
29.5-30.7 |
17.5-8.7 |
|
23 |
L3D, L3T |
L3 |
Thermolicer |
4 (7) |
2.5-3.1 |
118-174 |
Aug-21 |
5-6 |
12 |
30.5 |
18.5 |
|
24 |
L3T, L3D |
L3 |
Thermolicer |
7 |
3.6-4.7 |
69-111 |
Nov-21 |
5-6 |
7.1 |
29.5-30.3 |
22.4-23.2 |
|
25 |
L7D |
L7 |
Thermolicer |
6 |
1.6-2.6 |
154-195 |
Sep-21 |
6-8 |
9.3 |
29.5-31.3 |
20.2-22 |
|
26 |
L7D |
L7 |
Thermolicer |
5 |
2.4-3.5 |
164-199 |
Oct-21 |
3-6 |
7.5 |
31.0-31.3 |
23.5-23.8 |
|
27 |
L7D |
L7 |
Thermolicer |
5 |
3.0-4.2 |
163-198 |
Nov-21 |
3-5 |
5.8 |
28.7-29.6 |
22.9-23.8 |
|
N. |
Groups |
Farm |
Method |
# |
Weight (kg) |
Fish (x1000) |
Date |
FD |
C |
Bar |
|---|---|---|---|---|---|---|---|---|---|---|
|
28 |
L2D1, L2T, L2D2 |
L2 |
Hydrolicer |
8 |
2.1-4.1 |
94-195 |
Jun-21 |
4-5 |
6.8 |
|
|
29 |
L5D |
L5b |
Hydrolicer |
7 |
4.9-5.2 |
84-88 |
Oct-21 |
3-4 |
8.0 |
1.58-1.62 |
|
30 |
L6T1 |
L6a |
Hydrolicer |
5 |
2.6-3.6 |
83-91 |
Oct-21 |
4-7 |
7.5 |
1.24-1.26 |
|
31 |
L6T2, L6T1 |
L6b |
Hydrolicer |
9 |
3.4-4.5 |
66-94 |
Oct-21 |
4-7 |
5.9 |
1.24-1.42 |
|
32 |
L3D, L3T |
L3 |
Hydrolicer |
9 |
2.7-4.3 |
67-113 |
Sep-21 |
2-5 |
10.2 |
|
|
33 |
L10T |
L10 |
Hydrolicer |
4 |
1.7-1.8 |
115-142 |
Oct-21 |
6-7 |
7.7 |
1.00-1.15 |
2.8 - Harvest and total production data
At the harvest plant, after gutting and bleeding, each fish is measured for weight and graded into quality classes (Superior, Ordinary, Production, and Reject). Superior is defined as a premium product without significant defects and a positive overall impression, and Ordinary and Production are progressively lower grades of product while Reject fish are unusable due to sexual maturation, excessive deformities, wounds, damage, or other defects. The companies could therefore provide cage level data about the exact number and average weight of fish harvested for each quality class. Together with the production data, this was used to calculate biological feed conversion ratio (amount of feed used per kg fish) (FCR), and economic feed factor (amount of feed used per kg fish at harvest) (EFCR).
3 - Results
3.1 - Fish mortality after transfer
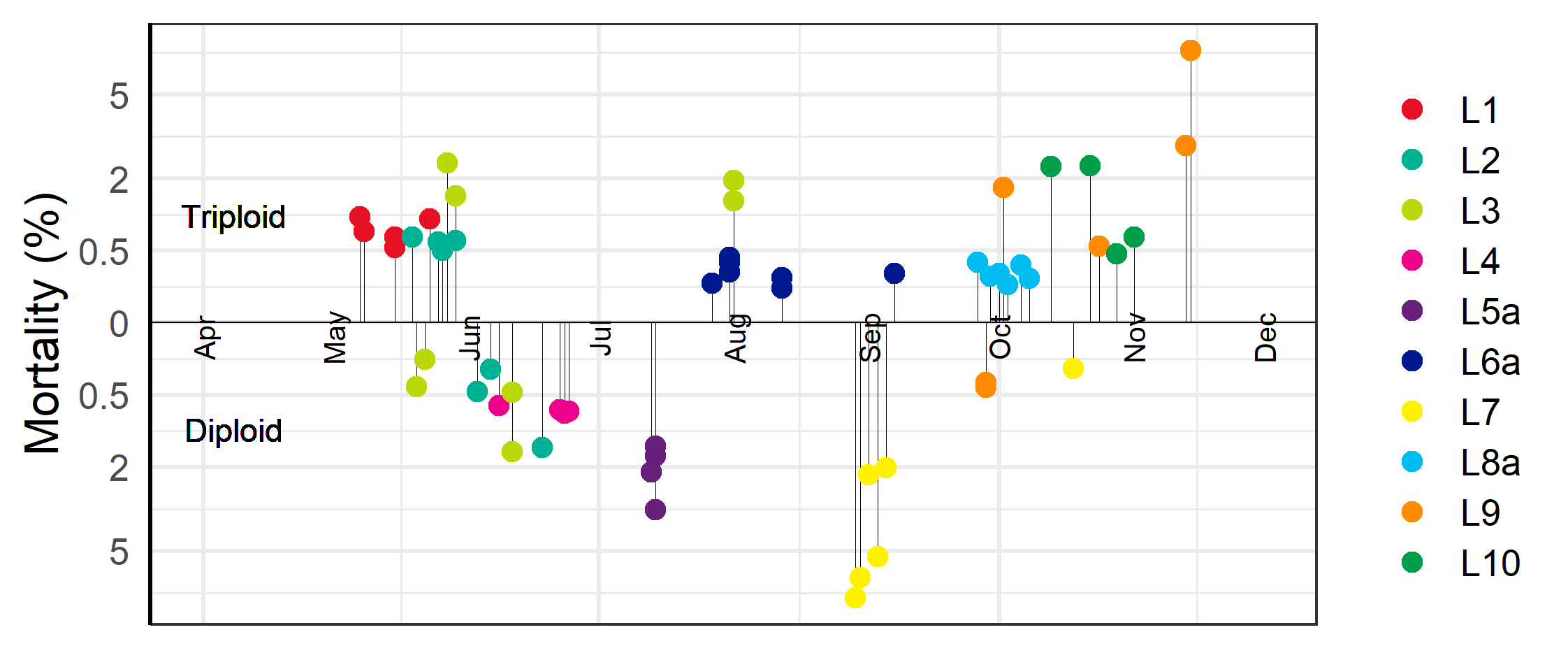
Average accumulated mortality one month after transfer was 1.1 ± 0.4 % (mean ± SE) for the triploid vs. 1.4 ± 0.5% for the diploid groups (p=0.68, Welch two Sample t-test) The high average mortality for the diploid was mainly caused by the transfers at L5a and at L7 (Figure 1). L5a received one group of diploid salmon mid-July (group L5D, Table 1). The mortality the days after transfer was high, and accumulated mortality 1.6 to 3.6 % after 1 month pending cage (Figure 1). The fish health service reported that it observed many fish with seriously damaged pelvic fins, and that many of the dead fish had wounds and spots of bleeding on the liver. The degree of internal damage led them to suspect bacterial sepsis which they believed likely would have been present at the hatchery of origin. The transfer of diploid fish to L7 (group L7D) in August-September had accumulated mortality from 2.1 to 7.4 % one month after transfer (Figure 1). Investigation by the fish health service revealed that the fish was positive for both Infectious Pancreatic Necrosis (IPN) and Heart and Skeletal Muscle Inflammation (HSMI) and concluded that this together with the stress involved in having been transferred from the hatchery to the sea cage had led to the high mortality. A late batch from the same hatchery group was delivered to the site mid-October (Table 1). This cage only got 0.2 % mortality after 1 month (Figure 1) and on their monthly visit, the fish health service deemed the health of these fish to be very good.
The high average mortality for the triploid was mainly due to the transfers at L3, L10 and L9 (Figure 1). The mortality at L3 was related to gill damage caused by an incident of high levels of organic material in the water at the hatchery. The two first cages with triploid fish delivered at L10 both displayed accumulated mortality around 2.4 % after 1 month at sea (Figure 1). However, the fish health service did not find anything wrong with the fish and suspected that the high mortality was due to damages received during the transport. L9 received groups of triploid salmon from two different hatcheries (Table 1). The first of these (L9T1) got mortality from 2.1 to 9.1 % mortality after 1 month (Figure 1). These fish had been diagnosed with HSMI at the hatchery, and although the disease had stabilized, it reoccurred again after sea transfer, and for two of the cages the mortality continued to be elevated reaching an accumulated amount of more than 20% after three and a half months, which resulted in them being terminated by the farmer. The second group of triploid fish (L9T2) at L9 came from another hatchery, and the cage with this fish only had 0.6 % mortality after 1 month in the sea and the two cages with diploid (group L9D) only had 0.4 % mortality (Figure 1).
3.2 - Mortality and ulcer development winter season, and healing towards summer
Mortality was higher during the first winter in the sea for triploid than for diploid fish with a linear regression of the log-transformed mortality indicating significant differences due to ploidy (Figure 2, p<0.001). The same model indicated significantly higher mortality for fish transferred to sea in the autumn season compared to the spring, with spring fish having relatively less mortality differences due to ploidy (Figure 2, p<0.001).
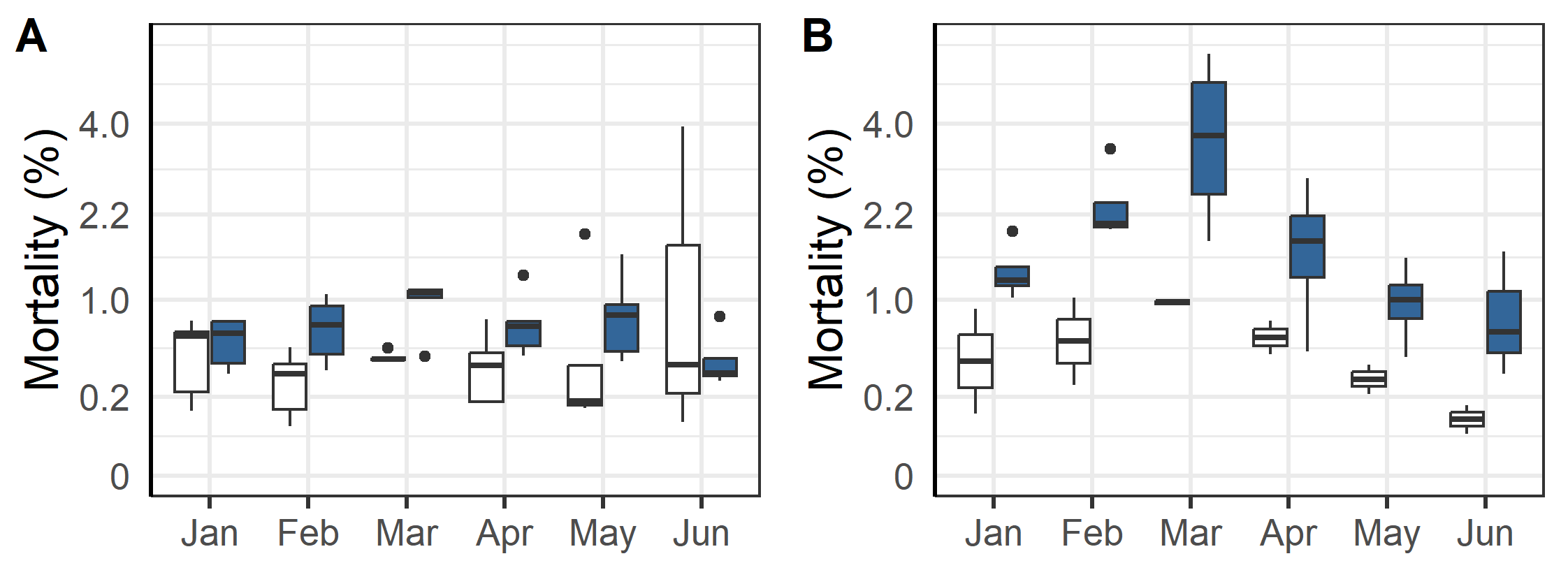
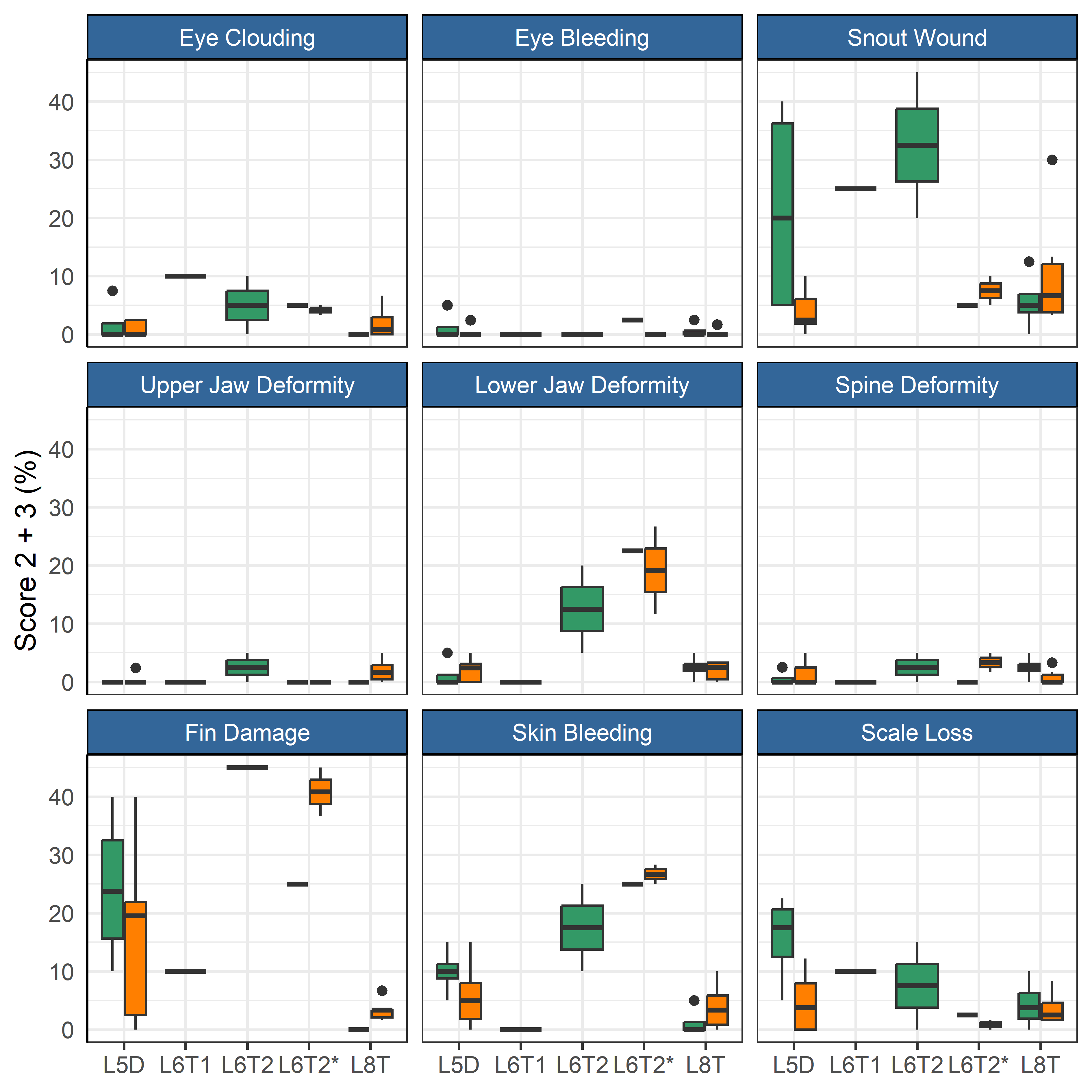
During the triploids first winter at sea one of the problems frequently reported by fish health personal was the prevalence of winter ulcers. These assessments were corroborated by a binomial statistical model of the weekly scores of external injuries which showed that triploid and autumn transferred fish had significantly more wounds and ulcers (Figure 3, p<0.001). Farmers responded to the occurrence of winter ulcers by euthanizing fish with wounds, by treating some of the effected cages with antibiotics, and by selectively terminating cages with poor welfare and high mortality (see below). Despite these efforts, the prevalence of wounds in the triploid cages did not improve until May and June when increasing temperatures and longer day-lengths facilitated healing.
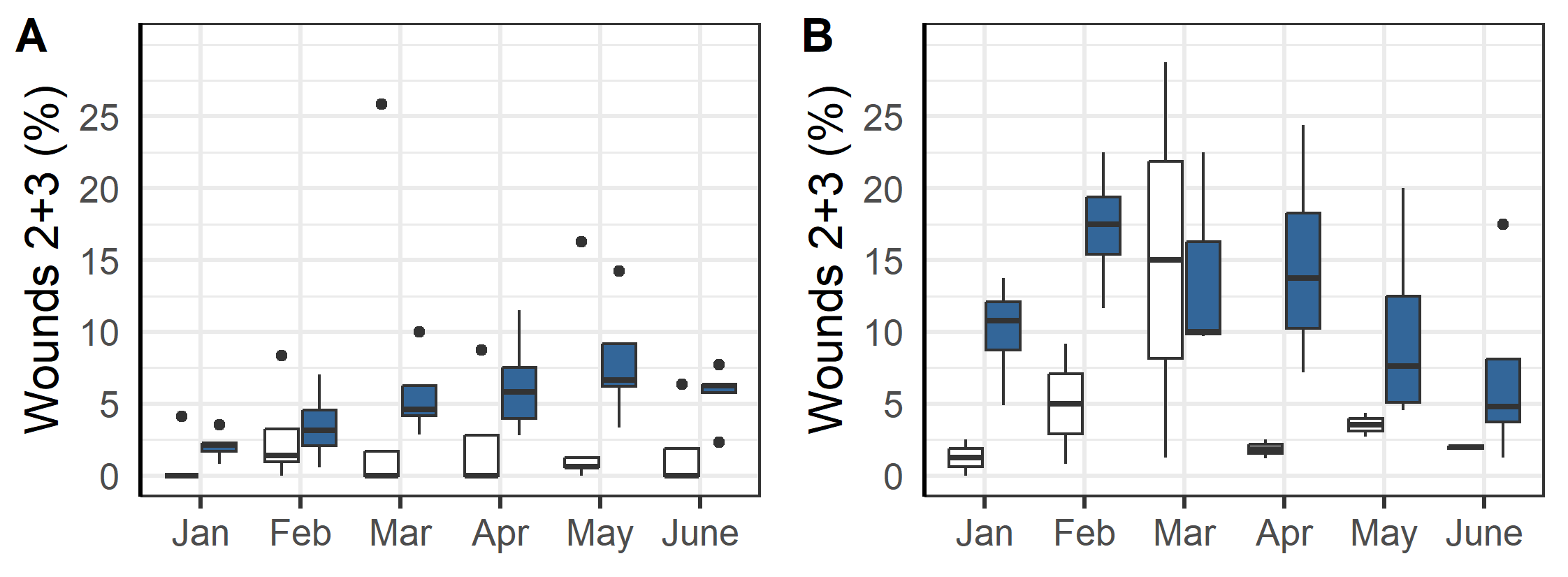
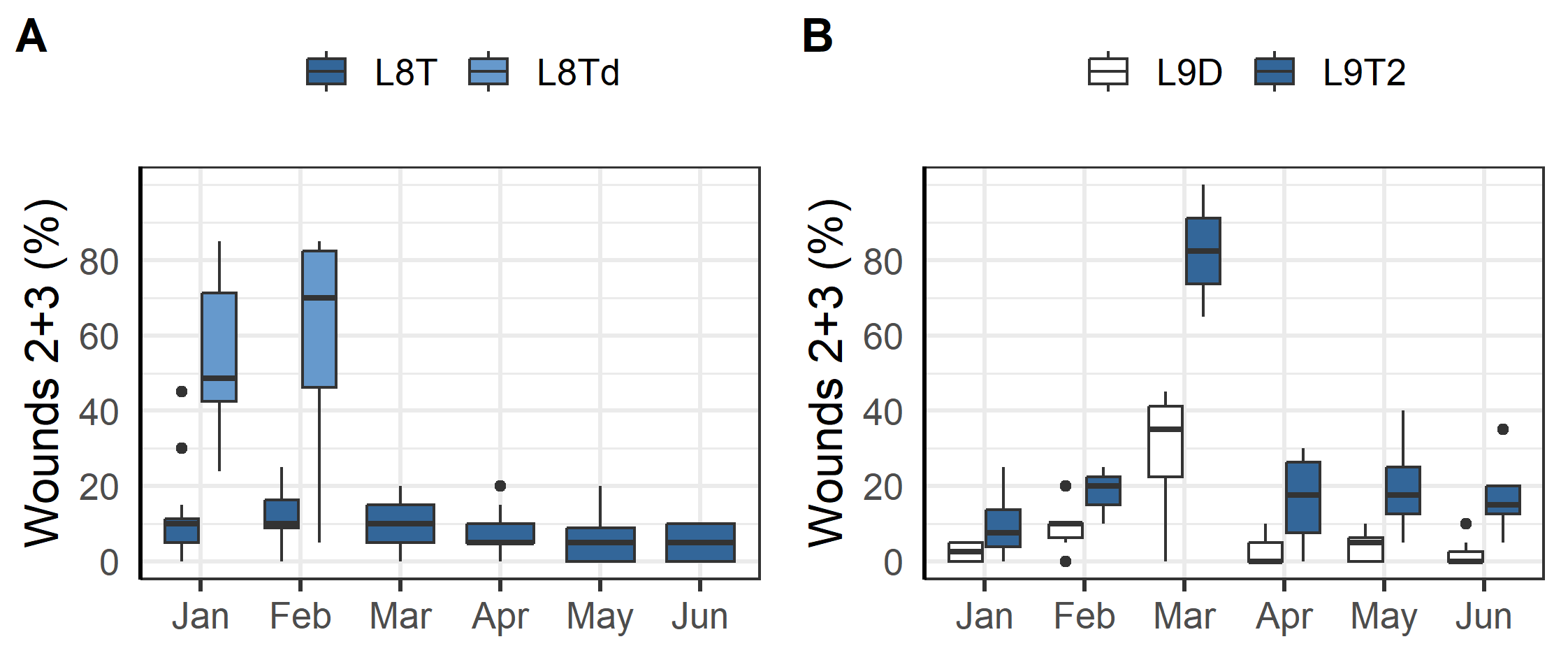
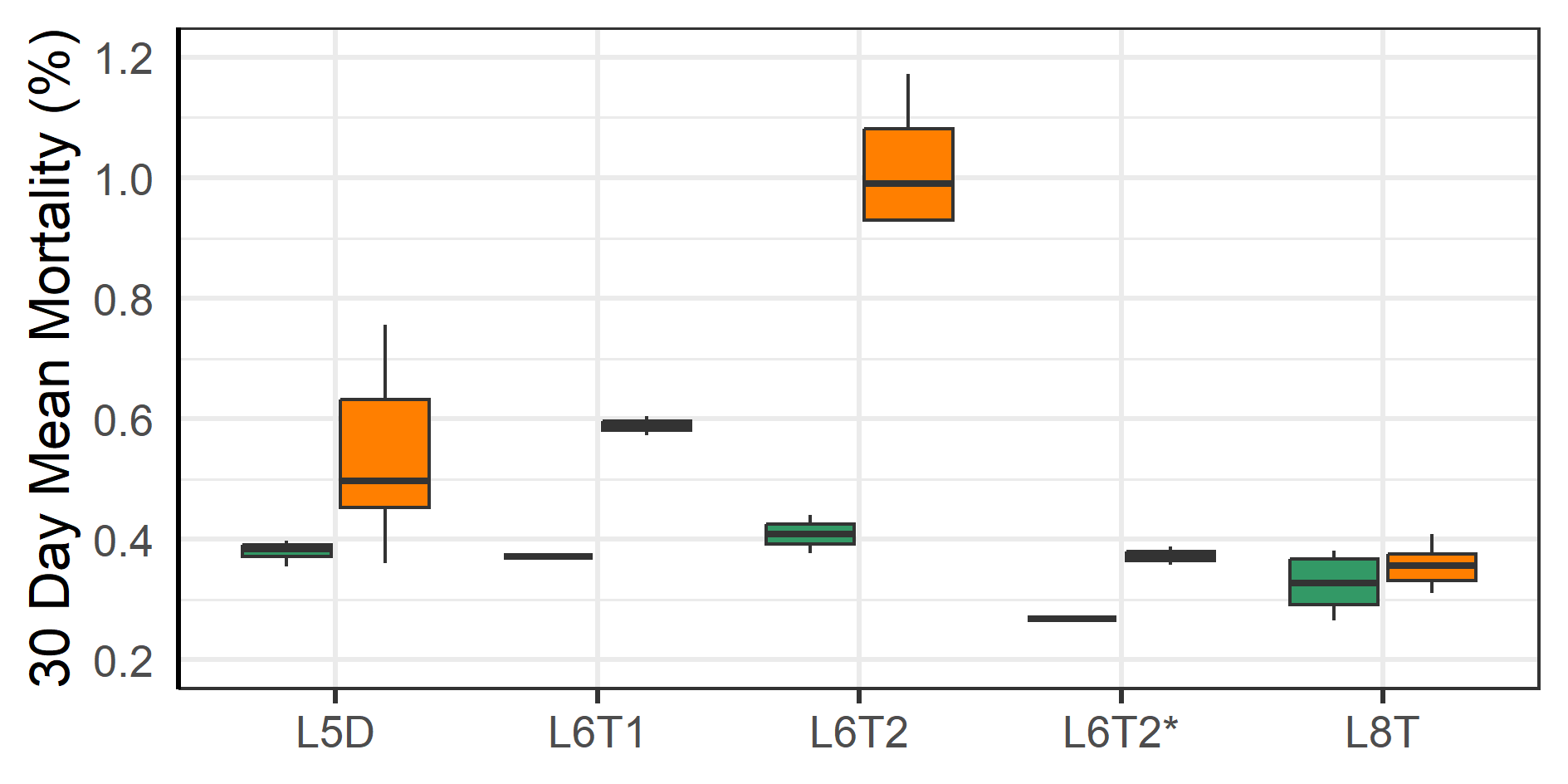
Outbreaks of winter ulcers were especially detrimental to two of the triploid fish groups at L8a and L9. The fish at L8a were transferred to sea in the autumn and during that first winter the prevalence of ulcers was elevated compared to other localities with two of the cages having more than 50% of sampled fish with scores of 2 or 3 (Figure 4A). It was therefore decided to terminate those cages, treat the remaining with antibiotics, and euthanise as many infected fish as possible. Seemingly these efforts mitigated the outbreak and in the following months there was no further increase in the prevalence of ulcers (group L8T, Figure 4A). Similarly, the fish at L9 were transferred to sea in the autumn and were affected by an outbreak of ulcers that first winter. The farmers responded by treating the fish with antibiotics in March and selectively euthanizing infected fish which improved the situation (Figure 4B).
3.3 - Fish Welfare before vs. after moving fish to new farm
After the first winter at sea three of the farms moved some or all their fish to other nearby farms (Table 2). These moves were associated with an increase in mortality for the month after the move compared to the month before (Figure 5). A linear regression model indicated that the mortality increase was significant (p = 0.005), but unrelated to ploidy (p = 0.82). The increase was smallest for the L6T* and 8T-group, which both were moved in June when sea temperature was moderate (9 °C), while the other moves were during periods of colder sea temperatures (see Table 2). These moves also had low mortality in the month before their respective moves (Figure 5). The dataset was however too small (too few cases) to do statistical analysis comparing effect from sea temperature or fasting time. The prevalence of injuries in welfare categories did not change for most categories (Figure 6), but those for fin damage, scale wounds, and snout wounds indicated a decreased frequency 30 days after transfer (Fisher exact test, respective p = 0.020, <0.001, <0.001).
3.4 - Fish welfare before vs. after delousing
The first delousings were done in late 2020 early 2021 at L5a, L2, L3 and L1, and then in June 2021 at L2 (Table 3-5). The majority of the delousings were done in the autumn of 2021 during a period when sea temperatures were elevated and lice pressure was high and they had to apply several delousing treatments in succession (Figure 7). For example, the triploid salmon at L10 (group L10T) was first deloused with mechanical and medicinal treatments in the autumn of 2021, then needed to be deloused again in January and March of 2022. For those latter delousing the farmer did not judge it safe for the fish to use mechanical or thermal delousing and instead opted for freshwater and medicinal treatments which were perceived to be gentler on the fish. In general, the delousing operations done in December 2021 were medicinal treatments, while mechanical and thermal were used the months before (Figure 7).
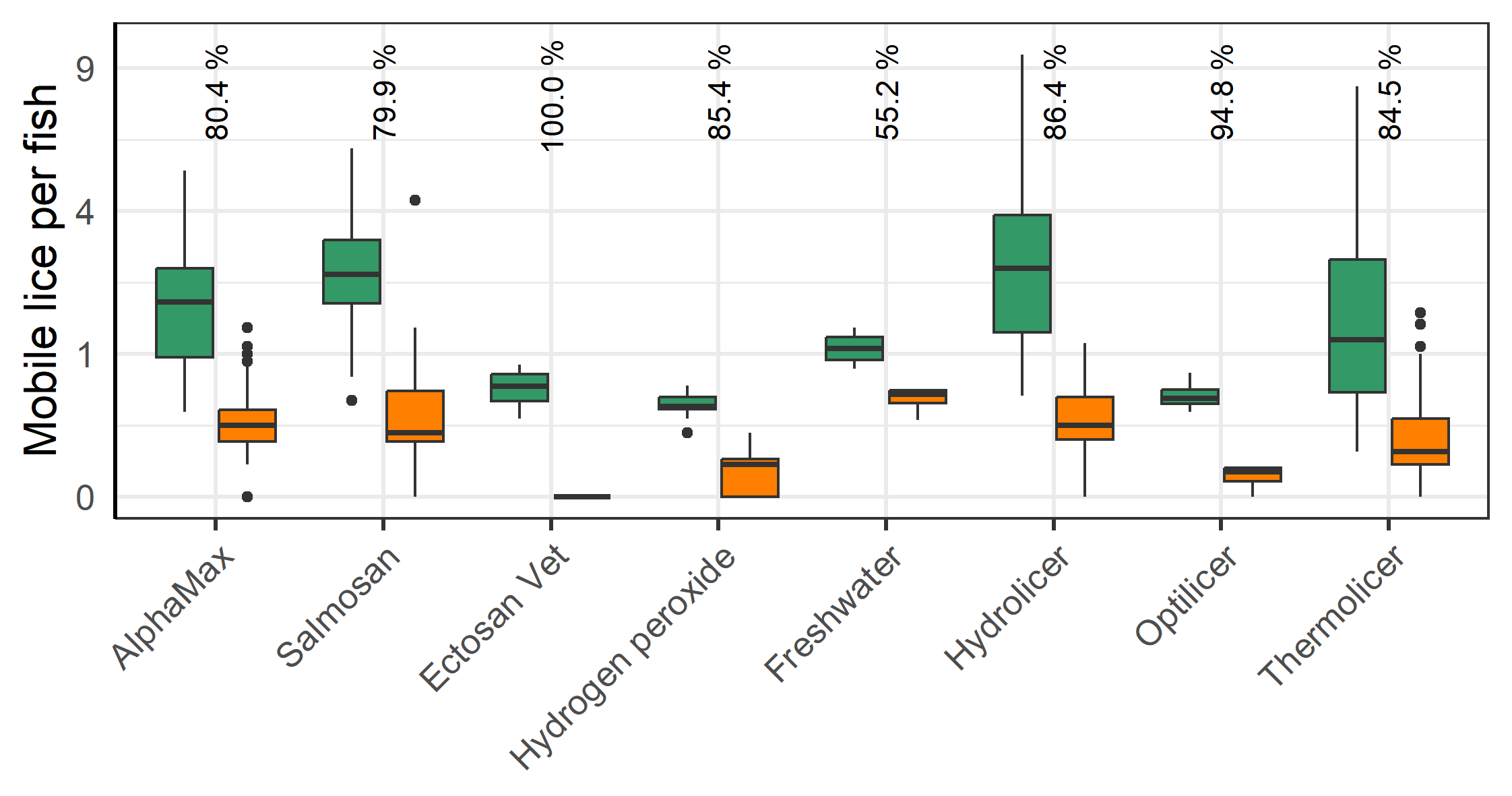
All methods lead to a significant reduction in mobile lice (Figure 8). Ectosan Vet, hydrogen peroxide, freshwater and Optilicer were only tried once at a single locality, albeit to multiple cages therein. Thus, confounding factors cannot be ruled out and the results of these treatments are therefore not readily comparable to others. The medicinal treatments of AlphaMax and Salmosan, along with the mechanical Hydrolicer and warm water Thermolicer treatments all had similar efficacies in removing mobile stages of lice (p>0.7, TukeyHSD). It should here be noted, that while freshwater and medicinal treatments have relatively good efficacy against sessile stages (Powell et al., 2015), thermal and mechanical treatments are less effective (Grøntvedt et al., 2015; Roth, 2016; Andrews & Horsberg, 2021; Nilsson, Barrett, et al., 2022).
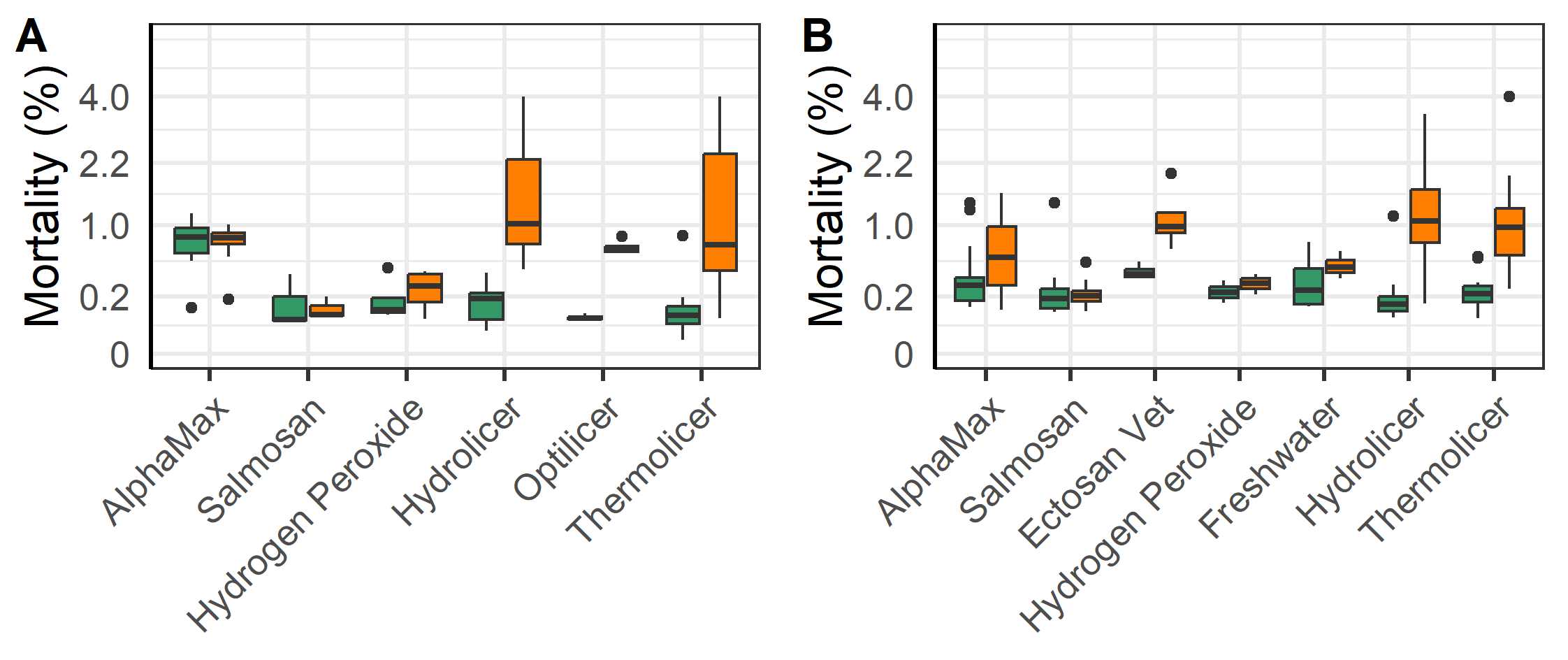
According to linear regression models of the log-transformed mortality, there was an increase in mortality for all fish following mechanical and thermal treatments, but with a significantly greater increase for the triploid salmon. (p<0.001, Figure 9). Likewise, there was a lesser but increased mortality following chemical and freshwater treatments (p = 0.013, Figure 9), with the triploid salmon having a slighter greater increase in mortality (p = 0.002).
3.5 - Fasting before delousing
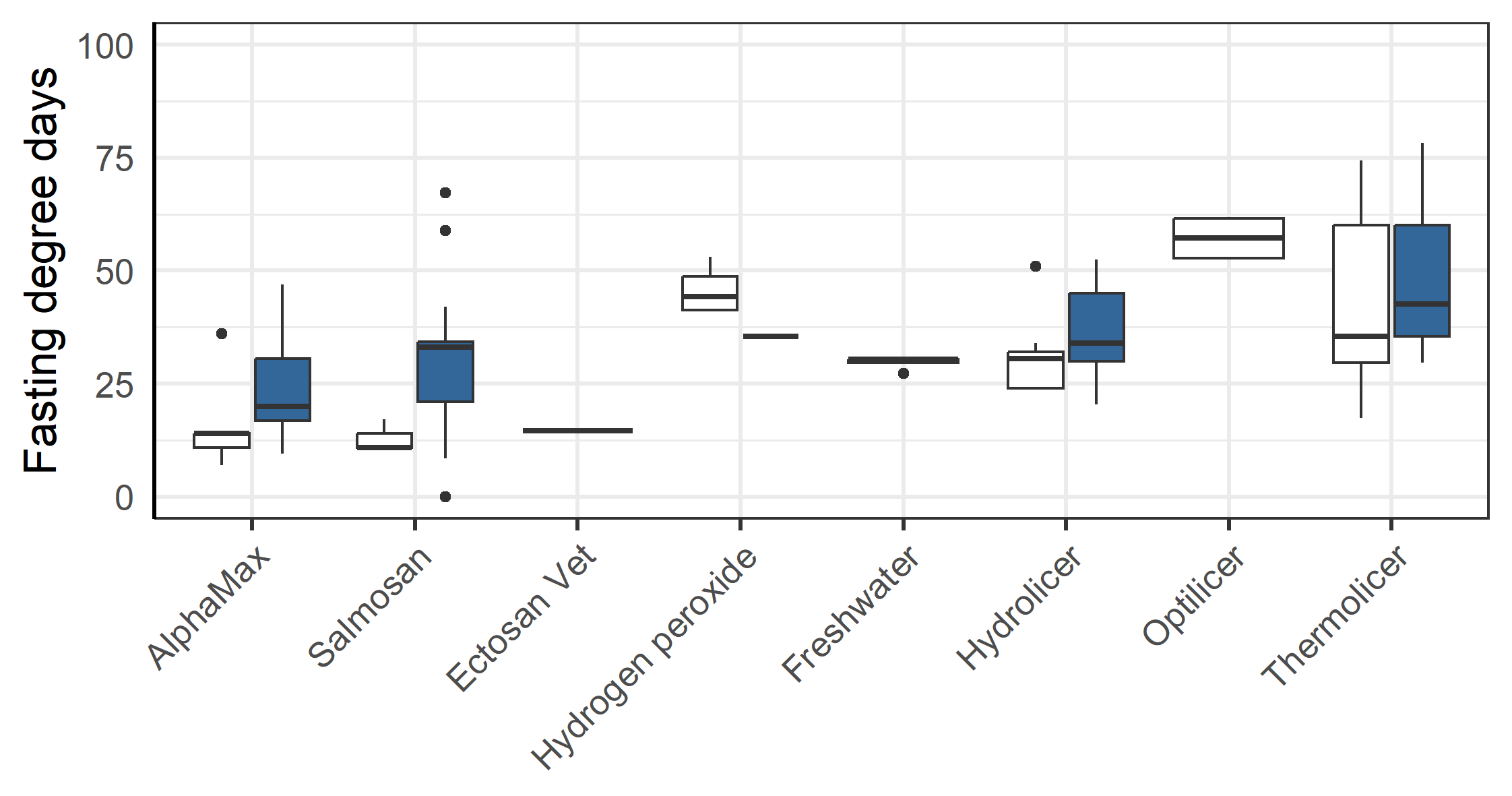
There was a clear pattern in that the farmer chose to fast the triploid fish longer than the diploid groups before delousing treatments (p=0.011, anova, Figure 10). This difference was especially large before treatments with AlphaMax and Salmosan (p=0.005, anova, Figure 10). There was also a clear pattern in that the fish were fasted for less degree days by the farmer before AlpaMax and Salmosan treatments that typically were done in tarpaulin (see Table 3), vs. the other methods that required the fish to be pumped into a well-boat (p<0.001, anova).
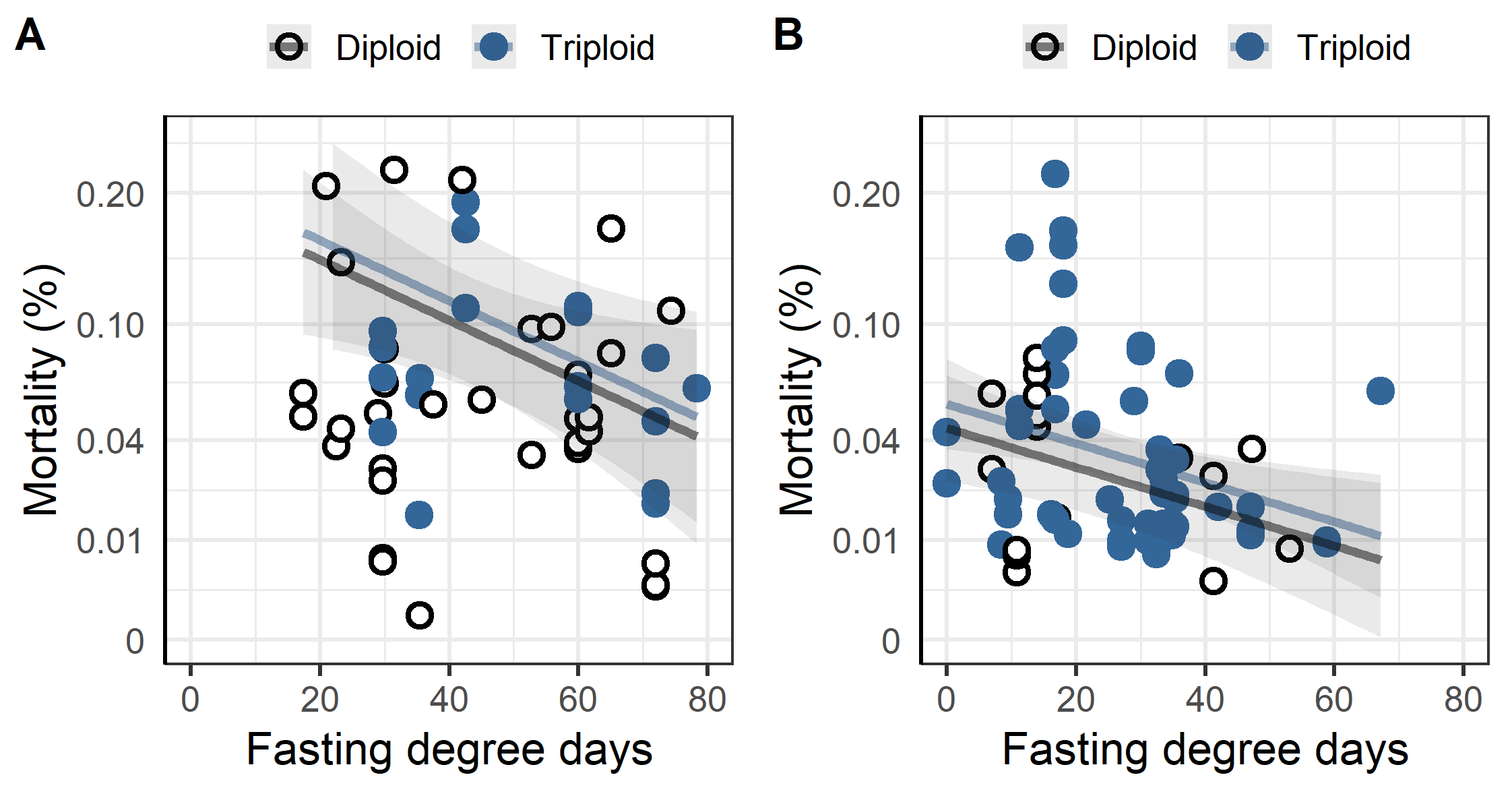
When a thermal or chemical bath treatment (Alphamax, Salmosan, Hydrogen peroxide) was applied increased fasting time significantly decreased the mortality in the week following treatment (Figure 11). According to the linear regression every 10-degree days decreased the sqrt mortality by 0.03% for thermal treatments (p = 0.021), and by 0.02% for chemical bath treatments (p = 0.008). Ploidy was not a significant factor in either treatment group.
3.6 - Decision to harvest
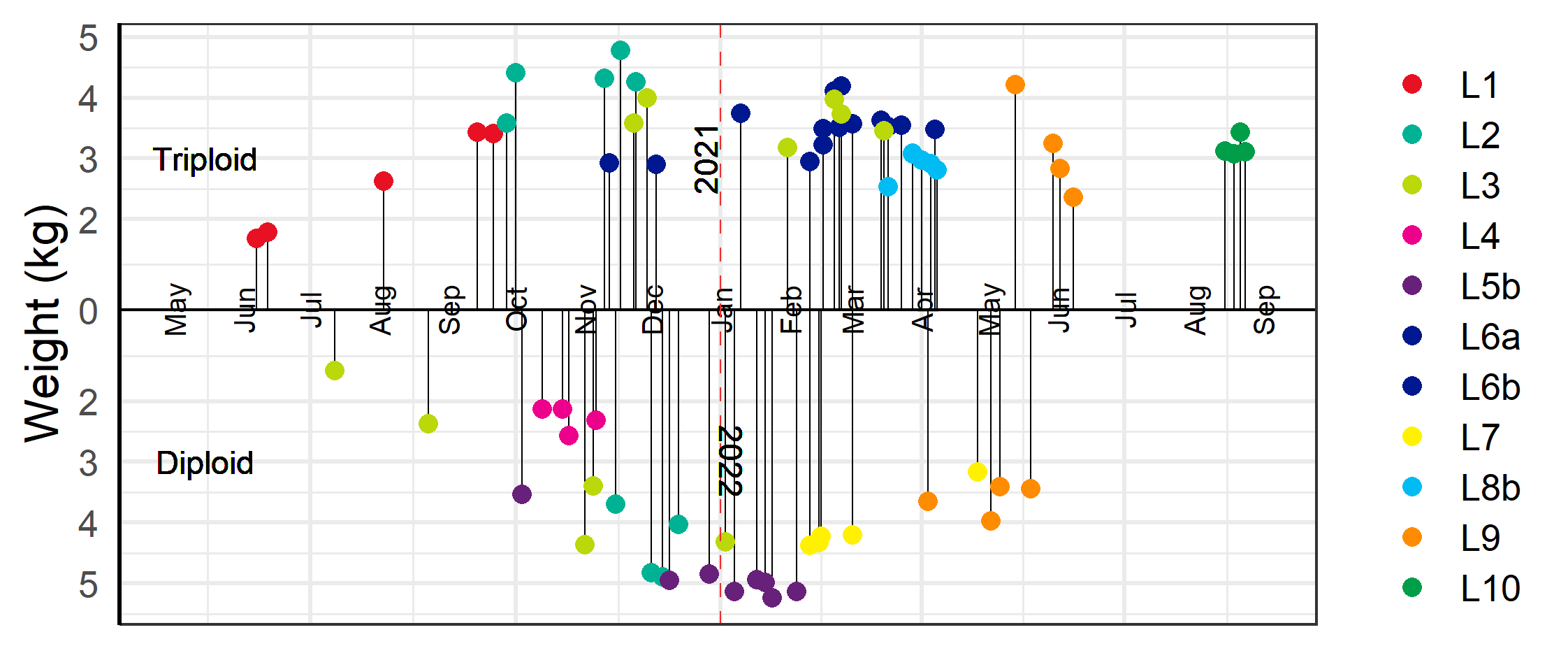
Three of the ten farm sites (L1, L2, and L4) were diagnosed with ISA (Table 5), and the farmers were therefore obliged to harvest these fish as soon as possible. While the two triploid ISA outbreaks had known pathways for infection the diploid outbreak had no nearby farm with known ISA outbreak. The first outbreak among the fish groups investigated here occurred at L1 which had only triploid fish (group L1T). A neighbouring site (LX), not included in this study, was diagnosed with ISA in October of 2020. The following February of 2021 some of the tests for ISA-virus were positive at L1, but the disease was not confirmed until May 2021. Two of the L1 cages with confirmed ISA were harvested in June 2021 at a low slaughter weight, and the other cages were slaughtered as they reached more economically viable slaughter weights above 4 kg, with the final cage slaughtered in September 2021 (Figure 12). The second outbreak of ISA was at L4 with only diploid fish (group L4D). This outbreak was unrelated to the first, and with no known infection in neighbouring farms. The site was diagnosed in June 2021, and the fish slaughtered at only 3 kg in October 2021 (Figure 12). In the same month the triploid group at the neighbouring site L2 (group L2T) was also diagnosed with ISA, and in November the diploid groups (groups L2D1 and L2D2) at this site had clinical indications of ISA. Fortunately for the farmers, the fish were relatively large, and the farmers had already started harvesting (Figure 12).
|
Hatchery |
Group |
Farm |
Wu |
Ten |
Nep |
HSMI |
CMS |
Su |
Parv |
IPN |
ISA |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
Ha1 |
L1T |
L1 |
X |
X |
X |
||||||
|
Ha2 |
L2T |
L2 |
X |
X |
X |
||||||
|
Ha5 |
L2D1 |
L2 |
X |
X |
|||||||
|
Ha7 |
L2D2 |
L2 |
X |
X |
|||||||
|
Ha3 |
L3D |
L3 |
X |
X |
X |
||||||
|
Ha4 |
L3T |
L3 |
X |
X |
X |
||||||
|
Ha6 |
L4D |
L4 |
X |
X |
X |
X |
|||||
|
Ha8 |
L5D |
L5a |
X |
X |
X |
||||||
|
Ha2 |
L6T1 |
L6a – L6b |
X |
X |
X |
||||||
|
Ha2 |
L6T2 |
L6a – L6b |
X |
X |
|||||||
|
Ha7 |
L7D |
L7 |
X |
X |
X |
X |
|||||
|
Ha2 |
L8T |
L8a-L8b |
X |
X |
|||||||
|
Ha9 |
L9D |
L9 |
X |
||||||||
|
Ha4 |
L9T1 |
L9 |
X |
X |
|||||||
|
Ha2 |
L9T2 |
L9 |
X |
X |
|||||||
|
Ha1 |
L10T |
L10 |
X |
X |
X |
Various other pathogens and diseases were widespread among both the diploid and triploid groups which required treatment and early slaughter in some cases (Table 5). The diploid group L3D were plagued with a CMS-HSMI co-infection and two of the worst infected cages were pre-emptively slaughtered in July 2021 at a weight below 3 kg, while the other cages, including cages with triploid fish (group L3T), were slaughtered later at higher weights (Figure 12). The farms at L5b, L6a and L6b, and L7 all needed to delouse cages several times during the autumn which led to increased prevalence of injured fish and outbreak of winter ulcers during the winter season (see sections 4.2 & 4.4). Ultimately it was decided to slaughter the affected fish early at L6a and L6b when fish weights were near 4 kg. The diploid fish at L5b (group L5D) and L7 (group L7D) were deemed to have less severe outbreaks of winter-ulcers and it was therefore decided to harvest later at larger sizes with two of the L7 cages harvested as late as May 2022 (Figure 12).
In June of 2021, six cages of triploid fish from L8a (group L8T) were moved to L8b (Table 2). The following spring lice numbers at the site were increasing, the prevalence of snout injuries was observed to be high among sampled fish, and overall health of the fish was perceived to be poor. It was therefore decided to slaughter the fish at relatively low weights in March and May of 2022 rather than risk delousing weak fish which might result in high mortality and increased injuries (Figure 12).
At the L9 farm site, the diploid group of fish (group L9D) were slaughtered between April and May 2022, while the triploid fish (groups L9T1 and L9T2) were slaughtered between May and June (Figure 12). The diploid fish had been transferred to sea earlier than the triploids (Table 1) and by the time of slaughter they had reached harvestable sizes. Meanwhile, both groups of triploid fish were put to sea later in the autumn and the following winter had been infected with an outbreak of winter-ulcers along with an unconfirmed outbreak of CMS which resulted in an elevated cumulative mortality. Thus, they were harvested somewhat earlier than desired with a lower harvest weight. The triploid fish at L10 (group 10T) was harvested August 2022. At this time the fish health service judged the health condition of the fish to be good and there were no health or welfare related reasons behind the decision to slaughter.
3.7 - Production performance
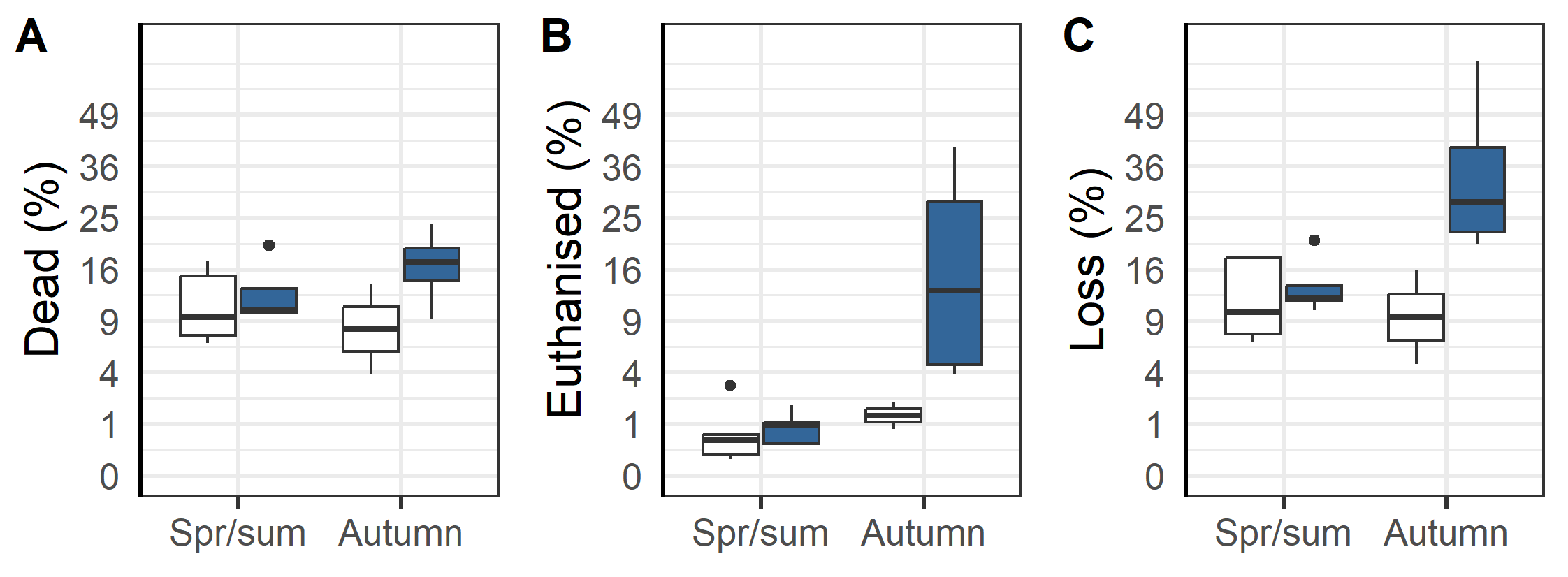
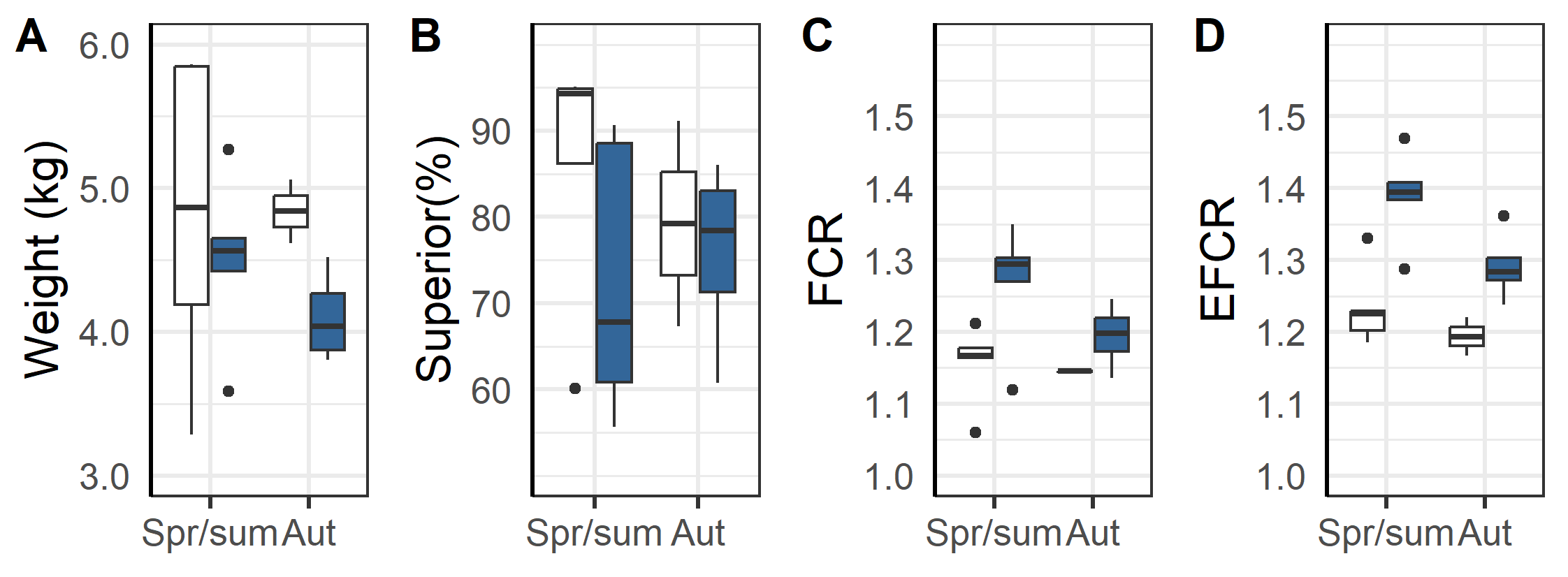
An overall evaluation of the 2020 generation of salmon production indicates that the triploids had higher percentage dead, euthanized and total loss than the diploid groups (Figure 13 and 14). The triploid had higher total loss (23.3%±5.7 % vs. 11.6±2.1%, p=0.04). The total losses were similar for triploids and diploids put to sea in the Spring/Summer (13.6±1.9% vs. 12.1±2.5 %, p=0.32, t-test), while those triploid fish transferred in the Autumn had much higher rates of loss compared to diploids (35.4±10.2 % vs. 10.3±5.6 %, p < 0.05, t-test). These differences persist despite the triploid fish being slaughtered earlier in their production cycle and at lower weights (Figure 14 A). It was also found that the triploid fish were less likely to be classified as superior quality (Figure 14 B) and that triploid fish required higher biological (Figure 14 C) and economic feed factor (1.35±0.03 vs. 1.22±0.02, p<0.001, t-test, Figure 14D), regardless of whether they were spring/summer or autumn transferred (Figure 14 C & D). Altogether and despite the removal of four culled cages from the dataset used in these calculations, the results suggest that compared to diploids the cultivation of triploids is economically inferior.
4 - Discussion
4.1 - Complex network
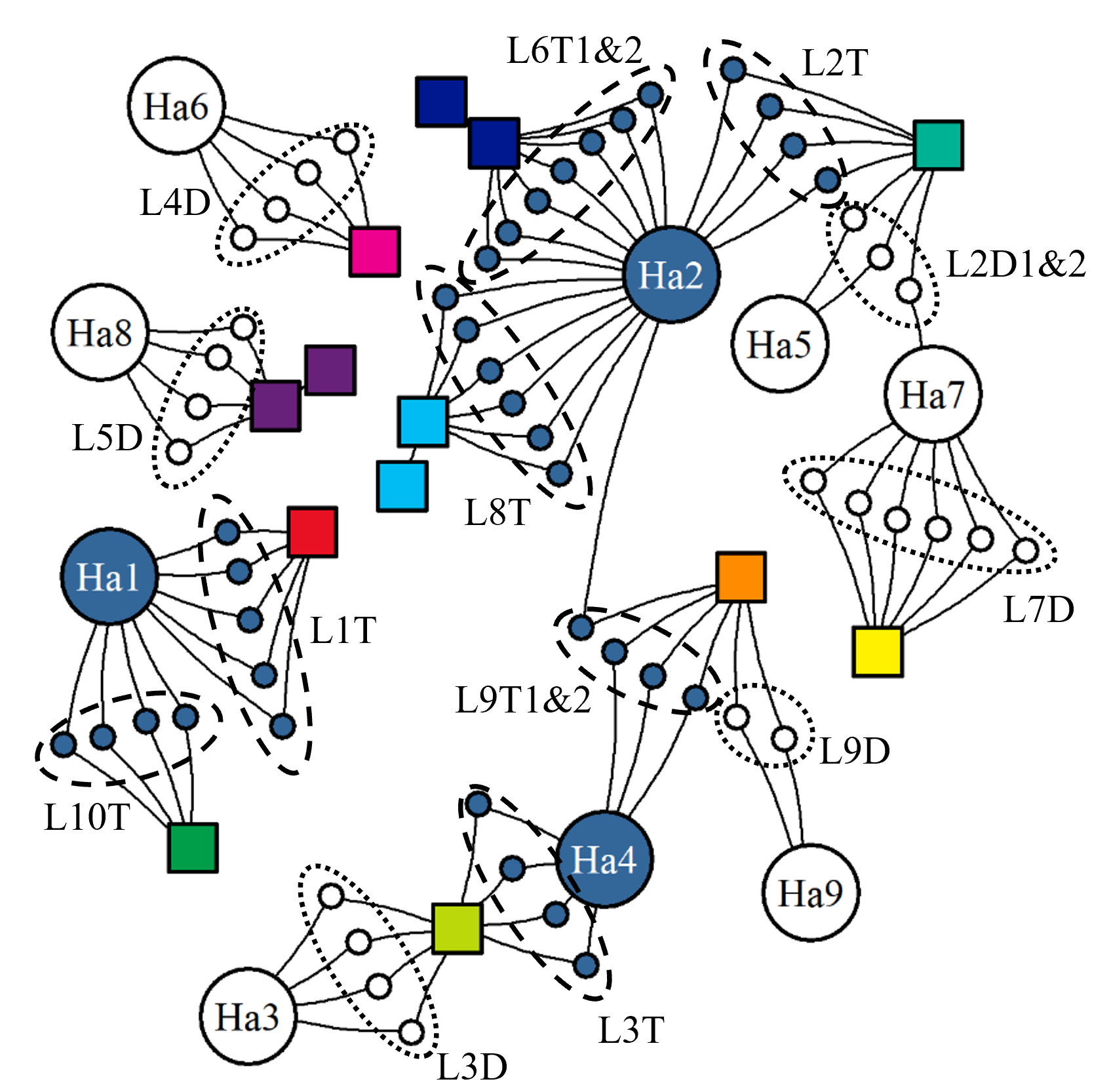
When evaluating this commercial production data, it is important to remember that unlike in a controlled trial the fish here are from numerous egg batches of differing strains, from several hatcheries, and have various histories. These fish groups are being cultivated within an aquaculture network that follows commercial logic and various regulations which limit the scope of the actions taken (Figure 15). Thus, all production cycles of fish groups, the operations carried out, and the environments experienced will vary.
4.2 - Forced to cull triploids
Post-transfer mortality of fish is dependent on their specific conditions and histories as illustrated by three cases of diploid and triploid fish having health problems originating from their respective hatcheries. This is also observed in the industry at large, and the Norwegian Food Safety Authorities together with the Veterinary institute recently issued a statement highlighting this problem (Norwegian Food Safety Authorities and the Norwegian Veterinary Institute, 2022). Interestingly two of these cases were fish groups with HSMI-outbreak, of which one of the groups were diploid (group L7D) and the other triploid (group L9T1). In the latter case the HSMI infection combined with transfer was so fatal for the triploid fish that it resulted in the farmer deciding to terminate cages, this did not become necessary for the diploid fish group. However, these two groups are incomparable, isolated cases and one can therefore not conclude based on these cases alone. HSMI is one of the most common diagnoses in Norwegian Salmon farming (Sommerset et al., 2022) and is normally only associated with mass mortality in connection with handling events stressing the fish. It is possible that triploids added vulnerably to stress also makes them more vulnerable to HSMI. On the other hand CMS-HSMI co-infection led to a decision of early harvest at a weight below 3 kg for one of the diploid groups (group L3D), while the triploid group at this site did not have an HSMI-outbreak. On the other hand, CMS-HSMI co-infection led to a decision of early harvest at a weight below 3 kg for one of the diploid groups (group L3D), while the triploid group at this site did not have an HSMI-outbreak.
A more convincing increased vulnerability to disease of triploids compared to diploids is demonstrated by their higher prevalence of winter-ulcers and higher mortality during their first winter at sea, and this also conforms to the observations from previous generations (Stien et al., 2019; Stien et al., 2021ab). In this investigation of the 2020 generation, two of the triploid groups transferred in the autumn had severe enough outbreaks of winter-ulcers that it was necessary to treat the fish with antibiotics and terminate some of the cages. The problem of winter-ulcers is exacerbated by the long winters in northern Norway where sea temperatures do not start rising until May-June. Generally, the wound healing process in salmon is very temperature dependent and slow at the low temperatures experienced in Northern Norway during the winter (Jensen et al., 2015). Once the sea-temperature increases past 8°C the salmon are better able to heal wounds and fight off infections (Lunder et al., 1995).
Farmers and fish health personnel have proposed several hypotheses for explaining why triploid salmon have more winter ulcers. They report that triploid fish are more reactive to stimuli which leads them to jump more and accidentally injure themselves, and they hypothesise that the resulting wounds become infected by opportunistic bacteria such as Moritella viscosa. Another hypothesis is that the triploid fish have more fragile skin and thereby more easily get damaged, and/or that they have inferior ability for wound healing. It has been speculated that the large blood cells in triploids compared to diploids result in poorer blood flow in peripheral capillaries and thereby poorer wound healing (Aunsmo et al., 2022). These hypotheses should be followed up with additional investigations.
4.3 - Disease forcing early harvest
Unfortunately, three of the decisions to end production and harvest the fish were due to ISA outbreaks. Results from previous generations have indicated higher risk of ISA in triploid than in diploid (Stien et al., 2019; Stien et al. 2021ab; Aunsmo et al., 2022). However, for the 2020 generation presented here, ISA was as prevalent in the triploid fish groups (1T and 2T) as the diploid fish groups (2D1, 2D2, and 4D). Nevertheless, the descriptions of the specific outbreaks suggest that when there is an increase in infection pressure due to a neighbouring outbreak of ISA, the triploid fish are more likely to be infected themselves. None of the fish groups in the project have so far been vaccinated against ISA, the high prevalence and consequence of ISA recommends that vaccination should be considered for future generations of both ploidies.
Besides ISA which warrants harvest by law, disease and perceived welfare status of the fish often influenced the decision to slaughter, especially if the fish were due to be deloused. This is in line with a national trend, where early slaughtered often is preferred to risky delousings (Barrett et al., 2022). The decision to slaughter fish earlier than planned also results in the production having a lower cumulative mortality than if production had continued as usual. From a fish health and welfare perspective this is clearly a good thing, but it is a decision that also confounds the mortality data and may lead to wrong conclusions if not carefully considered.
4.4 - Triploids give poor economy compared to diploids
The triploid groups put to sea in the autumn had significant higher mortality, rates of euthanasia and total loss compared to the diploid, while the triploid groups put to sea in the spring/summer had similar losses as the diploid transferred to sea in the same period. These numbers thereby suggest that triploid can be produced with similar mortality numbers as diploid. However, the weight of the diploid fish at harvest was in many cases much higher and implies that the mortality numbers do not reflect underlying differences in the production of diploids and triploids. This conclusion is further supported by the triploid fish at harvest having a significantly lower percentage of fish classified as superior. Considering that higher weight typically means higher price per kg, and that fish classified as superior typically get higher price this means that the farmer received a lower price per kg triploid than per kg diploid fish. Furthermore, the farmer had a higher cost in feed per kg triploid produced compared to diploid. Altogether these findings show that triploids are economically inferior for the farmer.
4.5 - Consequences of moving fish
The data shows that moving fish to a new farm can lead to mortality. This is not surprising, as moving fish will necessarily involve first crowding the fish, then pumping them into a well boat, transportation and finally pumping the fish into a new cage at a new farm. All operations that are known to stress and risk harming the fish (Overton et al., 2018; Oliveira et al., 2021; Walde et al., 2021; Persson et al., 2022). The current dataset shows much higher mortality for the three moves that were done at low temperature (4-6°C), compared to two that were done at moderate temperature (9°C). The limited number of cases makes it challenging to confidently attribute the mortality difference to temperature either fully or partially. Nevertheless, here we see that the salmon’s ability to cope with stress decreases substantially at 4-6 °C compared to at 9°C. The difference in coping ability to these moves could also be attributed to a greater prevalence of wounded individuals in the colder fish groups who contribute to the higher post move mortality. This hypothesis is supported by the observation that wound prevalence decreased after the move which suggests that the wounded and weakened individuals are the ones who die.
4.6 - Fish welfare at delousing
Delousing operations are a known hazard to salmon and currently one of, if not the, major drivers of mortality and poor welfare outcomes in Norwegian salmon farming (Grefsrud et al., 2022). The farmer faces the dilemma of having to abide by a strict lice limit while simultaneously adhering to welfare regulations (Stien et al., 2020; Macaulay et al., 2022). A farmer will seek to limit the amounts of delousing operations to limit the welfare impact on the fish and allow for as much time between the treatments as possible to enable the salmon to recover. However, during the autumn of 2021 the lice pressure was high and several of the fish groups needed to be deloused 3-4 times within a short timeframe. Typically, the farmers first chose to delouse the salmon using non-medicinal methods and then for the latter treatments they preferred chemical treatments. The non-medicinal treatments are known to have a greater negative impact on the fish (Overton et al., 2019, Grefsrud et al., 2022), and so the farmers are preferentially using them when the fish are perceived to be stronger. The farmers are then able to use the gentler chemical methods on the salmon when they are believed to be weaker and more susceptible to injury and death. Importantly, the number of chemical applications allowed at any one fish group is restricted to maximum 3 per production cycle under the green licenses (Ministry of Trade, Industry and Fisheries, 2013). The farmers choice of delousing method and application timing reflects how they adapt their husbandry practices to match the state of the fish but also how their decisions are shaped by the regulatory regime.
The farmer’s choice of delousing treatment also considers the efficacy of the various methods. The findings here show that all methods except for the freshwater baths were highly effective at removing mobile stages. However, the freshwater treatment was tried only once and there are multiple factors which influence its efficacy including the salinity level and bath duration (Powell et al., 2015). While all treatments can effectively remove mobile stages, the thermal and mechanical methods are less effective at removing sessile stages than the various chemical treatments including freshwater (Powell et al., 2015; Nilsson et al., 2022b). Unfortunately, salmon lice have developed resistance to medicinal and hydrogen peroxide treatments, and plausibly could develop resistance to freshwater treatments (Powell et al., 2015; Nilsson, Barrett, et al., 2015; Nilsson et al., 2022b). Farmers and the fish health personnel that advise them are aware of this and make their choices within farms and areas accordingly.
Mechanical and thermal treatments are typically chosen in periods with relative warm sea temperatures when the fishes wound healing ability is high. Meanwhile, the chemical treatments, which have a lesser risk of injury, are preferred when the temperatures are low. Another advantage of the chemical methods is that they remove sessile lice. In choosing chemical methods at the end of the autumn the farmers hope to avoid additional delousings before spring and avoid doing delousings in the winter when cold temperatures make the salmon especially vulnerable. Ectosan Vet. (imidakloprid) presents a special case because it is a newly available chemical method with 100% efficacy, but it is costly, must be applied in a well boat, demands treatment water cleaning and is therefore not broadly applied.
Where post treatment salmon mortality intersects with the delousing method used and ploidy of the fish group, we see the influence of the farmers' choices. It is probable that farmers generally choose methods they believed to be less risky for fish that were vulnerable, for example AlphaMax was used to treat the diploid fish which had the highest pre-treatment mortality in our dataset. Farmers preferentially used the gentler chemical bath methods on triploid fish while diploid groups were more often treated with mechanical and thermal methods. Otherwise, there was little difference in the observed mortality following delousing operations between diploid and triploid groups of fish. This may again be due to farmers choices. Previous reports have shown that triploid have higher proportion of fish with jaw deformation and at higher risk to die during crowding and delousing (Stien et al., 2021ab). The farmers also report that they perceive the triploid fish as more skittish and less tolerant to stress. In the cases where there were both triploid and diploid fish at the farm, their policy was to always delouse the cages with diploid fish first. This allowed the personnel to train and test the equipment before starting with the fish they perceived as more fragile, the triploids.
Overall mechanical and thermal methods had higher mortality post vs. pre-treatment compared to the traditional chemical methods (AlphaMax and Salmosan). The diploids treated with these methods had no increase in mortality, which could be explained by the use of tarpaulins which crowd fish but are much less impactful than pumping fish into well-boats. Chemical baths in tarpaulins are the preferred choice for fish welfare reasons but they also allow the treatment chemical to be released into the environment where it may harm non-target animals, specifically the commercially important shrimp Pandalus borealis. Thus, the practice has been restricted in recent years which means the fish must be treated in well-boats, and that switch may partially explain a recent increase in mortality (Grefsrud et al., 2022).
4.7 - Fasting before delousing
The evidence suggests that farmers and fish health personnel decide on the length of fasting prior to treatment based on the perceived risk to the fish. Specifically, the perceived weakness of triploids resulted in their longer fasting time for some chemical treatments and the greater impact of thermal and mechanical treatments led to longer fasting times for all fish. These choices are interesting since there is little previously reported scientific evidence comparing effect on fasting length before delousing. However, farmers have personal experience which leads them to their decisions and companies can conduct their own internal investigations, the results of which would be proprietary and go unpublished. The possible link with less risk of mortality with increasing fasting degree days found here is therefore novel. The result should however be interpreted with care as there may be other factors, the feeding was for instance typically turned off on the same day, and then the cages deloused sequentially, meaning that the farmer would have gained experience delousing these fish using this specific equipment along the way, possibly reducing the risk for the latter treatments. Therefore, these findings of benefits from fasting which are based on industry data should be followed up with controlled experiments to test the hypotheses.
5 - Conclusions
5.1 - The 2020-generation in review
The sampled triploid salmon in the 2020-generation had generally higher incidence of lower jaw deformities (5.2 vs. 0.4%, Table 6), snout wounds (12.3 vs. 6.9%, Table 6), skin wounds (6.8 vs 2.3%, Table 6) and higher total loss (23.3 vs. 11.6%, Table 6). Although the triploid and diploid groups in the 2020-generation are not directly comparable with each other (not control groups with identical background reared at the same farms), the results are consistent and in agreement with what has been seen for the 2014-2019 generations (Stien et al., 2019, 2021ab). The weekly registrations of wound status in the fish also support that triploids are slower to heal than diploids. The results for the 2020-generation of triploid salmon therefore confirm that triploid salmon is inferior to diploid salmon in relation to fish health and welfare. Similarly, the 2020 generation also confirm that triploid salmon transferred to the sea in the autumn are at the greatest risk of high mortality and that triploid salmon are more vulnerable to ISA than diploid.
5.2 - Conclusions for the 2020-generation
This report differs from the previous (Stien et al., 2019, 2021ab) with novel investigations into the effects of handling and fasting, the influence of farm and fish health personnel’s decisions on the health and welfare of the fish, and the production performance in terms of result at slaughter and economic feed factor. The results show that handling is a risk to both diploid and triploid fish. One important observation here is that the farmers and the fish health personnel view the triploid as inferior and less robust than diploid and make adjustment to accommodate this. For instance, they prefer chemical delousing methods for triploids (Table 6), fast the triploids longer (more degree days) before delousing operations, and report that they make extra adjustments to optimize the operations for triploids. The added fasting and added caution around delousing triploids probably mitigate some of the fish health and welfare problems of the triploid and explain why the triploid salmon groups in the 2020-generation only had a small (but significant) added increase in mortality after delousing operations compared to diploid groups undergoing the same treatment types.
Another important finding was that the triploid salmon had higher economic feed conversation ratio (on average 1.35 in triploids vs 1.22 in diploid groups, Table 6), lower weight at slaughter (4.4 vs. 4.8 kg, Table 6) and less percentage of superior graded fish at slaughter (74 vs. 84%, Table 6). Considering that they also need specially designed, more costly feed, this means that triploids are higher cost and less profitable for the farmer than diploids while also having inferior health and welfare outcomes.
|
Gr. |
Transfer |
Diseases |
Ljd |
Snw |
Skw |
Months |
Treatments |
Loss |
EFCR |
Weight (kg) |
Sup |
|
L1T |
May |
Wu, Nep., ISA |
1% |
6% |
4% |
13-16 |
C |
12% |
1.39 |
2.7-4.4 |
84-95% |
|
L2D1 |
June |
HSMI, ISA |
0% |
6% |
1% |
18-19 |
M,T |
7% |
1.22 |
4.7-5.0 |
86% |
|
L2D2 |
June |
HSMI, ISA |
0% |
4% |
0% |
18 |
M |
8% |
1.20 |
5.8-5.9 |
93-97% |
|
L2T |
May |
Wu, HSMI, ISA |
0% |
13% |
3% |
16-18 |
M,T |
14% |
1.47 |
4.6-5.8 |
81-95% |
|
L3D |
May - Jun |
HSMI, CSM, Parv. |
0% |
0% |
0% |
13-20 |
H,T, M,T,C |
21% |
1.19 |
2.5-5.4 |
88-98% |
|
L3T |
May – Aug |
Wu, Su.,Parv, |
4% |
4% |
3% |
18-21 |
H,T,M,T,C |
21% |
1.29 |
4.2-5.0 |
57-78% |
|
L4D |
June |
Wu, HSMI, Parv, ISA |
0% |
2% |
0% |
16-17 |
T |
10% |
1.23 |
3.1-3.6 |
89-99% |
|
L5D |
July |
Wu, Ten, HSMI |
2% |
10% |
7% |
15-19 |
T,T,M,C |
18% |
1.33 |
4.5-6.2 |
52-83% |
|
L6T1 |
July – Sep |
Wu, Ten, Parv |
12% |
13% |
7% |
16-20 |
C,M,C |
12% |
1.45 |
4.5-5.1 |
47-71% |
|
L6T2 |
July |
Wu, Ten |
12% |
11% |
7% |
16-20 |
C,M,C |
10% |
1.38 |
3.9-5.2 |
43-77% |
|
L7D |
Aug.–Oct. |
Wu, HSMI, Parv, IPN |
0% |
8% |
3% |
16-19 |
T,T,T |
16% |
1.22 |
4.2-5.4 |
55-76% |
|
L8T* |
Sep.–Oct. |
Wu, Ten |
4% |
20% |
8% |
18 (5) |
C,C |
41% |
1.24 |
3.5-4.1 |
79-86% |
|
L9D |
Sep. |
Wu |
1% |
18% |
5% |
18-20 |
T,T,C |
5% |
1.17 |
4.4-5.0 |
88-93% |
|
L9T1* |
Oct.–Nov. |
Wu, HSMI |
5% |
12% |
10% |
20 (4) |
T,T,C |
74% |
1.28 |
3.8-5.2 |
55-66% |
|
L9T2 |
Oct. |
Wu, CSM |
5% |
17% |
11% |
19-20 |
T,T,C |
20% |
1.28 |
3.4-4.3 |
70-80% |
|
L10T |
Oct.–Nov. |
Wu, CSM, Parv |
4% |
15% |
8% |
22-23 |
M,C,F,C |
23% |
1.36 |
4.1-4.4 |
80-91% |
6 - References
Aaen SM, Helgesen KO, Bakke MJ, Kaur K, Horsberg TE, 2015. Drug resistance in sea lice: a threat to salmonid aquaculture. Trends Parasitol, 31(2), 72-81.
Aldrin M, Huseby RB, Jensen BB, Jansen, MD, 2021. Evaluating effects of different control strategies for infectious salmon anaemia (ISA) in marine salmonid farming by scenario simulation using a disease transmission model. Preventive Veterinary Medicine, 191, 105360.
Andrews M, Horsberg TE, 2021. In vitro bioassay methods to test the efficacy of thermal treatment on the salmon louse, Lepeophtheirus salmonis. Aquaculture, 532, 736013.
Aunsmo A, Martinsen L, Bruheim T, Sekkelsten‐Kindt MM, Sandtrø A, Gaasø S, Braaen S, Rimstad E, 2022. Triploid Atlantic salmon (Salmo salar) may have increased risk of primary field outbreaks of infectious salmon anaemia. Journal of Fish Diseases, 45(11), 1733-1743.
Barrett L, Oldham T, Kristiansen TS, Oppedal F, Stien LH, 2022. Declining size-at-harvest in Norwegian salmon aquaculture: Lice, disease, and the role of stunboats. Aquaculture, 738440.
Benfey TJ 2016. Effectiveness of triploidy as a management tool for reproductive containment of farmed fish: Atlantic salmon (Salmo salar) as a case study. Reviews in Aquaculture, 8(3), 264-282.
Benfey TJ, Sutterlin AM, 1984a. Growth and gonadal development in triploid landlocked Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences, 41(9), 1387-1392.
Benfey TJ, Sutterlin AM, 1984b. Triploidy induced by heat shock and hydrostatic pressure in landlocked Atlantic salmon (Salmo salar L.). Aquaculture, 36(4), 359-367.
Borge O, Bratlie S, Mellegård H, 2018. Proposal for relaxation of European regulations for deliberate release of genetically modified organisms (GMO). In: Bioteknologirådet (The Norwegian Biotechnology Advisory Board).
Brunsvik PS, 1997. Miljømessig avlusing av laks. Småtrykk 261, Gildeskål Forsøksstasjon AS.
De Fonseka R, Fjelldal PG, Sambraus F, Nilsen TO, Remø SC, Stien LH, Reinardy HC, Madaro A, Hansen TJ, Fraser TW, 2022. Triploidy leads to a mismatch of smoltification biomarkers in the gill and differences in the optimal salinity for post-smolt growth in Atlantic salmon. Aquaculture, 546, 737350.
FAO-FIGIS. (2021). Fisheries Global Information System (FAO-FIGIS). FAO Fisheries Division.
Fjelldal PG, Wennevik V, Fleming I, Hansen T, Glover K, 2014. Triploid (sterile) farmed Atlantic salmon males attempt to spawn with wild females. Aquaculture Environment Interactions, 5(2), 155-162.
Fraser TW, Fjelldal PG, Hansen T, Mayer I, 2012. Welfare considerations of triploid fish. Reviews in Fisheries Science, 20(4), 192-211.
Golpour A, Siddique MAM, Siqueira-Silva DH, & Pšenička M, 2016. Induced sterility in fish and its potential and challenges for aquaculture and germ cell transplantation technology: a review. Biologia, 71(8), 853-864.
Grefsrud ES, Bjørn PA, Grøsvik BE, Hansen PK, Husa V, Karlsen Ø, Kvamme BO, Samuelsen O, Sandlund N, Solberg MS, Stien LH, 2022. Risikorapport norsk fiskeoppdrett 2022 – kunnskapsstatus — Effekter på miljø og dyrevelferd i norsk fiskeoppdrett. Rapport fra havforskningen; 2022-13.
Grøntvedt RN, Nerbøvik I-KG, Viljugrein H, Lillehaug A, Nilsen H., Gjerve, A, 2015. Termisk avlusing av laksefisk–dokumentasjon av fiskevelferd og effekt. Rapport 13, 2015. Veterinærinstituttets rapportserie.
Gullestad P, Bjørgo S, Eithun I, Ervik A, Gudding R, Hansen H, Johansen R, Osland A, Rødseth M, Røsvik I, 2011. Effektiv og bærekraftig arealbruk i havbruksnæringen–areal til begjær. Oslo
Hersoug B. 2021. Why and how to regulate Norwegian salmon production? – The history of Maximum Allowable Biomass (MAB). Aquaculture, 545, 737144.
Hvas M, Stien LH, Oppedal F, 2021. The effect of fasting period on swimming performance, blood parameters and stress recovery in Atlantic salmon post smolts. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 255, 110913.
Jensen LB, Wahli T, McGurk, Eriksen TB, Obach A, Waagbø R, Handler A, Tafalla C 2015. Effect of temperature and diet on wound healing in Atlantic salmon (Salmo salar L.). Fish Physiology and Biochemistry 41, 1527-1543
Kumar G, Engle CR, 2016. Technological Advances that Led to Growth of Shrimp, Salmon, and Tilapia Farming. Reviews in Fisheries Science & Aquaculture, 24(2), 136-152.
Lunder T, Evensen Ø, Holstad G, Håstein T, 1995. ‘Winter ulcer’ in the Atlantic salmon Salmo salar. Pathological and bacteriological investigations and transmission experiments. Diseases of aquatic Organisms 23, 39-49.
Macaulay G, Barrett LT, Dempster T, 2022. Recognising trade-offs between welfare and environmental outcomes in aquaculture will enable good decisions. Aquaculture Environment Interactions, 14, 219-227.
Madaro A, Kjøglum S, Hansen T, Fjelldal PG, Stien LH, 2021. A comparison of triploid and diploid Atlantic salmon (Salmo salar) performance and welfare under commercial farming conditions in Norway. Journal of Applied Aquaculture, 1-15.
Midtlyng PJ, Grave K, Horsberg TE, 2011. What has been done to minimize the use of antibacterial and antiparasitic drugs in Norwegian aquaculture? Aquaculture Research, 42, 28-34.
Ministry of Trade, Industry and Fisheries,2013. Forskrift om endring i forskrift om tildeling av løyve til havbruk med matfisk av laks, aure og regnbogeaure i sjøvatn i 2013 (Regulation on changes to regulations on the awarding of licenses for aquaculture with food fish of salmon, aure and rainbow aure in seawater in 2013).
Myhre Jensen E, Horsberg TE, Sevatdal S, Helgesen KO, 2020. Trends in de-lousing of Norwegian farmed salmon from 2000-2019-Consumption of medicines, salmon louse resistance and non-medicinal control methods. Plos one, 15(10), e0240894.
Nilsson J, Gismervik K, Nielsen KV, Iversen MH, Noble C, Kolarevic J, Frotjold H, Nilsen K, Wilkinson E, Klakegg B, 2022a. Laksvel-Standardisert operasjonell velferdsovervåking for laks i matfiskanlegg. Rapport fra havforskningen; 2022-14.
Nilsson J, Barrett LT, Mangor-Jensen A, Nola V, Harboe T, Folkedal O, 2022b. Effect of water temperature and exposure duration on detachment rate of salmon lice (Lepeophtheirus salmonis); testing the relevant thermal spectrum used for delousing. Aquaculture, 738879.
Norwegian Directorate of Fisheries (2022a). Aquaculture Statistics: Atlantic salmon and rainbow trout statistics. The Norwegian Directorate of Fisheries.
Norwegian Directorate of Fisheries (2022b). Grønne tillatelser. https://www.fiskeridir.no/Akvakultur/Tildeling-og-tillatelser/Kommersielle-tillatelser/Laks-oerret-og-regnbueoerret/Groenne-tillatelser. Accessed 2022-12-21
Norwegian Food Safety Authorities and Norwegian Veterinary Institute, 2022. Sykdomsoppklaring oppdrett. A written note/letter from the two institutions.
Oliveira VHS, Dean KR, Qviller L, Kirkeby C, Bang Jensen B, 2021. Factors associated with baseline mortality in Norwegian Atlantic salmon farming. Scientific reports, 11: 14702
Overton K, Dempster T, Oppedal F, Kristiansen TS, Gismervik K, Stien LH, 2019. Salmon lice treatments and salmon mortality in Norwegian aquaculture: a review. Reviews in Aquaculture, 11(4), 1398-1417.
Persson D, Nødtvedt A, Aunsmo A, Stormoen M, 2022. Analysing mortality patterns in salmon farming using daily cage registrations. Journal of Fish Diseases, 45(2), 335-347.
Powell, MD, Reynolds P, Kristensen T, 2015. Freshwater treatment of amoebic gill disease and sea-lice in seawater salmon production: Considerations of water chemistry and fish welfare in Norway. Aquaculture, 448, 18-28.
Roth B, 2016. Avlusing av laksefisk med Optilice: Effekt på avlusing og fiskevelferd. Nofima rapport nr. 59-2016, 41.
Sambraus F, Olsen RE, Remen M, Hansen TJ, Torgersen T, Fjelldal PG, 2017a. Water temperature and oxygen: The effect of triploidy on performance and metabolism in farmed Atlantic salmon (Salmo salar L.) post-smolts. Aquaculture, 473, 1-12.
Sambraus F, Fjelldal PG, Remø SC, Hevrøy EM, Nilsen TO, Thorsen A, Hansen TJ, Waagbø R 2017b. Water temperature and dietary histidine affect cataract formation in Atlantic salmon (Salmo salar L.) diploid and triploid yearling smolt. Journal of Fish Diseases, 40(9), 1195-1212.
Sambraus F, Remen M, Olsen RE, Hansen T, Waagbø R, Torgersen T, Lock E, Imsland A, Fraser T, Fjelldal, P, 2018. Changes in water temperature and oxygen: the effect of triploidy on performance and metabolism in large farmed Atlantic salmon. Aquaculture Environment Interactions, 10, 157-172.
Sambraus F, Hansen T, Daae BS, Thorsen A, Sandvik R, Stien LH, Fraser TW, Fjelldal PG, 2020. Triploid Atlantic salmon Salmo salar have a higher dietary phosphorus requirement for bone mineralization during early development. Journal of Fish Biology, 97(1), 137-147.
Sommerset I, Walde CS, Bang Jensen B, Wiik-Nielsen J, Bornø G, Oliveira VHS, Haukaas A og Brun E. Fiskehelserapporten 2021, Veterinærinstituttets rapportserie nr. 2a/2022, Norwegian Veterinary Institute
Stien LH, Tørud B, Gismervik K, Lien ME, Medaas C, Osmundsen T, Kristiansen TS, Størkersen KV, 2020. Governing the welfare of Norwegian farmed salmon: Three conflict cases. Marine Policy, 117, 103969.
Sønvisen SA, Vik C, 2021. Shaping Aquaculture Management—An Interest Tug O’ War. Sustainability, 13(16), 8853.
Taylor JF, Sambraus F, Mota-Velasco J, Guy DR, Hamilton A, Hunter D, Corrigan D, Migaud H, 2013. Ploidy and family effects on Atlantic salmon (Salmo salar) growth, deformity and harvest quality during a full commercial production cycle. Aquaculture, 410, 41-50.
Taylor JF, Waagbø R, Diez-Padrisa M, Campbell P, Walton J, Hunter D, Matthew C, Migaud H, 2015. Adult triploid Atlantic salmon (Salmo salar) have higher dietary histidine requirements to prevent cataract development in seawater. Aquaculture Nutrition, 21(1), 18-32.
Tiwary BK, Kirubagaran R, Ray AK, 2004. The biology of triploid fish. Reviews in fish biology and fisheries, 14(4), 391-402.
Torrissen O, Olsen RE, Toresen R, Hemre GI, Tacon AGJ, Asche F, Hardy RW, Lal S, 2011. Atlantic Salmon (Salmo salar): The “Super-Chicken” of the Sea? Reviews in Fisheries Science, 19(3), 257-278.
Waagbø R, Jørgensen SM, Timmerhaus G, Breck O, Olsvik PA, 2017. Short-term starvation at low temperature prior to harvest does not impact the health and acute stress response of adult Atlantic salmon. PeerJ, 5, e3273.
Walde CS, Bang Jensen B, Pettersen JM, Stormoen M, 2021. Estimating cage-level mortality distributions following different delousing treatments of Atlantic salmon (Salmo salar) in Norway. J Fish Dis, 44(7), 899-912.
